An Optimised Aqueous Extract of Phenolic Compounds from Bitter Melon with High Antioxidant Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Initial Solvent Extractions

2.4. Optimising the Aqueous Extraction
2.5. Extraction Efficiency
2.5.1. Total Phenolic Content
2.5.2. ABTS Assay
2.5.3. DPPH Assay
2.5.4. FRAP Assay
2.6. Statistical Analysis
3. Results
3.1. Initial Solvent Extractions


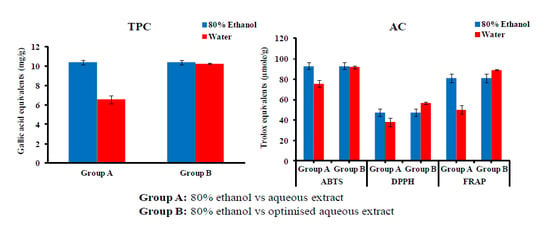
3.2. Optimising the Aqueous Extraction




| Chemical Properties | Aqueous Extract | 80% Ethanol Extract |
|---|---|---|
| TPC (mg GAE/g dry basis) | 10.6 ± 0.2 a | 10.7 ± 0.3 a |
| ABTS (μmol TE/g dry basis) | 94.8 ± 1.3 a | 95.7 ± 3.5 a |
| DPPH (μmol TE/g dry basis) | 58.6 ± 1.0 a | 49.0 ± 4.0 b |
| FRAP (μmol TE/g dry basis) | 91.9 ± 1.8 a | 83.5 ± 4.3 b |
| Correlation | ABTS | DPPH | FRAP |
|---|---|---|---|
| TPC | 0.830 ** | 0.784 ** | 0.922 ** |
| FRAP | 0.919 ** | 0.937 ** | |
| DPPH | 0.901 ** |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fang, E.F.; Zhang, C.Z.; Zhang, L.; Fong, W.P.; Ng, T.B. In vitro and in vivo anticarcinogenic effects of RNase MC2, a ribonuclease isolated from dietary bitter gourd, toward human liver cancer cells. Int. J. Biochem. Cell Biol. 2012, 44, 1351–1360. [Google Scholar] [CrossRef]
- Patel, S.; Patel, T.; Parmar, K.; Bhatt, Y.; Patel, Y.; Patel, N.M. Isolation, characterization and antimicrobial activity of charantin from Momordica charantia linn. fruit. Int. J. Drug Dev. Res. 2010, 2, 629–634. [Google Scholar]
- Hsu, C.; Tsai, T.-H.; Li, Y.-Y.; Wu, W.-H.; Huang, C.-J.; Tsai, P.-J. Wild bitter melon (Momordica charantia linn. var. abbreviata Ser.) extract and its bioactive components suppress Propionibacterium acnes-induced inflammation. Food Chem. 2012, 135, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Grover, J.K.; Yadav, S.P. Pharmacological actions and potential uses of Momordica charantia: A review. J. Ethnopharmacol. 2004, 93, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Sathishsekar, D.; Subramanian, S. Antioxidant properties of Momordica charantia (bitter gourd) seeds on streptozotocin induced diabetic rats. Asia Pac. J. Clin. Nutr. 2005, 14, 153–158. [Google Scholar] [PubMed]
- Semiz, A.; Sen, A. Antioxidant and chemoprotective properties of Momordica charantia L. (bitter melon) fruit extract. Afr. J. Biotechnol. 2007, 6, 273–277. [Google Scholar]
- Lii, C.K.; Chen, H.W.; Yun, W.T.; Liu, K.L. Suppressive effects of wild bitter gourd (Momordica charantia linn. var. abbreviata Ser.) fruit extracts on inflammatory responses in raw 264.7 macrophages. J. Ethnopharmacol. 2009, 122, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Tech. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Horax, R.; Hettiarachchy, N.; Islam, S. Total phenolic contents and phenolic acid constituents in 4 varieties of bitter melons (Momordica charantia) and antioxidant activities of their extracts. J. Food Sci. 2005, 70, C275–C280. [Google Scholar] [CrossRef]
- Horax, R.; Hettiarachchy, N.; Chen, P. Extraction, quantification, and antioxidant activities of phenolics from pericarp and seeds of bitter melons (Momordica charantia) harvested at three maturity stages (immature, mature, and ripe). J. Agric. Food Chem. 2010, 58, 4428–4433. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Ng, L.T. Antioxidant and free radical scavenging activities of wild bitter melon (Momordica charantia linn. var. abbreviata Ser.) in Taiwan. LWT-Food Sci. Technol. 2008, 41, 323–330. [Google Scholar] [CrossRef]
- Tan, S.P.; Parks, S.E.; Stathopoulos, C.E.; Roach, P.D. Greenhouse-grown bitter melon: Production and quality characteristics. J. Sci. Food Agric. 2014, 94, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Cerda, A.; Martínez, M.E.; Soto, C.; Poirrier, P.; Perez-Correa, J.R.; Vergara-Salinas, J.R.; Zúñiga, M.E. The enhancement of antioxidant compounds extracted from Thymus vulgaris using enzymes and the effect of extracting solvent. Food Chem. 2013, 139, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Negrão Murakami, A.N.; de Mello Castanho Amboni, R.D.; Prudêncio, E.S.; Amante, E.R.; de Moraes Zanotta, L.; Maraschin, M.; Cunha Petrus, J.C.; Teófilo, R.F. Concentration of phenolic compounds in aqueous mate (Ilex paraguariensis A. St. Hil) extract through nanofiltration. LWT-Food Sci. Technol. 2011, 44, 2211–2216. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 2008, 110, 881–890. [Google Scholar] [CrossRef]
- Meneses, N.G.T.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant capacity and phenolic content of selected tropical fruits from malaysia, extracted with different solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Golding, J.B.; Stathopoulos, C.E.; Nguyen, M.H.; Roach, P.D. Optimizing conditions for the extraction of catechins from green tea using hot water. J. Sep. Sci. 2011, 34, 3099–3106. [Google Scholar] [CrossRef] [PubMed]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive folin-ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Spigno, G.; de Faveri, D.M. Antioxidants from grape stalks and marc: Influence of extraction procedure on yield, purity and antioxidant power of the extracts. J. Food Eng. 2007, 78, 793–801. [Google Scholar] [CrossRef]
- Wissam, Z.; Ghada, B.; Wassim, A.; Warid, K. Effective extraction of polyphenols and proanthocyanidins from pomegranate’s peel. Int. J. Pharm. Pharm. Sci. 2012, 4, 675–682. [Google Scholar]
- Sólyom, K.; Solá, R.; Cocero, M.J.; Mato, R.B. Thermal degradation of grape marc polyphenols. Food Chem. 2014, 159, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Rispail, N.; Morris, P.; Webb, K. Phenolic compounds: Extraction and analysis. In Lotus Japonicus Handbook; Márquez, A., Ed.; Springer: Dordrecht, The Netherlands, 2005; pp. 349–354. [Google Scholar]
- Hirun, S.; Roach, P.D. An improved solvent extraction method for the analysis of catechins and caffeine in green tea. J. Food Nutr. Res. 2011, 50, 160–166. [Google Scholar]
- Nepote, V.; Grosso, N.R.; Guzmán, C.A. Optimization of extraction of phenolic antioxidants from peanut skins. J. Sci. Food Agric. 2005, 85, 33–38. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Hirun, S.; Roach, P.D.; Bowyer, M.C.; Phillips, P.A.; Scarlett, C.J. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Herbal Med. 2013, 3, 104–111. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Alli, I.; Ereifej, K.; Alhamad, M.N.; Alsaad, A.; Rababeh, T. Optimisation and characterisation of various extraction conditions of phenolic compounds and antioxidant activity in olive seeds. Nat. Prod. Res. 2011, 25, 876–889. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shi, L.; Fan, L.; Ding, Y.; Zhao, S.; Liu, Y.; Ma, C. Optimization of extraction and enrichment of phenolics from pomegranate (Punica granatum L.) leaves. Ind. Crop. Prod. 2013, 42, 587–594. [Google Scholar] [CrossRef]
- Gertenbach, D.D. Solid-liquid extraction technologies for manufacturing nutraceuticals. In Functional Foods: Bioachemical and Processing Aspects; Shi, J., Mazza, G., Maguer, M.L., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 332–365. [Google Scholar]
- You, Q.; Yin, X.; Zhang, S.; Jiang, Z. Extraction, purification, and antioxidant activities of polysaccharides from Tricholoma mongolicum Imai. Carbohydr. Polym. 2014, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.P.; Parks, S.E.; Stathopoulos, C.E.; Roach, P.D. Extraction of flavonoids from bitter melon. Food Nutr. Sci. 2014, 5, 458–465. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, Z.; Ma, H.; Atungulu, G.G. Extraction of phenolics from pomegranate peels. Open Food Sci. J. 2011, 5, 17–25. [Google Scholar] [CrossRef]
- Zu, G.; Zhang, R.; Yang, L.; Ma, C.; Zu, Y.; Wang, W.; Zhao, C. Ultrasound-assisted extraction of carnosic acid and rosmarinic acid using ionic liquid solution from Rosmarinus officinalis. Int. J. Mol. Sci. 2012, 13, 11027–11043. [Google Scholar] [CrossRef] [PubMed]
- Chiremba, C.; Rooney, L.W.; Beta, T. Microwave-assisted extraction of bound phenolic acids in bran and flour fractions from sorghum and maize cultivars varying in hardness. J. Agric. Food Chem. 2012, 60, 4735–4742. [Google Scholar] [CrossRef] [PubMed]
- Delsart, C.; Ghidossi, R.; Poupot, C.; Cholet, C.; Grimi, N.; Vorobiev, E.; Milisic, V.; Peuchot, M.M. Enhanced extraction of phenolic compounds from merlot grapes by pulsed electric field treatment. Am. J. Enol. Vitic. 2012, 63, 205–211. [Google Scholar] [CrossRef]
- Giannuzzo, A.N.; Boggetti, H.J.; Nazareno, M.A.; Mishima, H.T. Supercritical fluid extraction of naringin from the peel of Citrus paradisi. Phytochem. Anal. 2003, 14, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Ates, B.; Durmaz, G.; Yilmaz, I.; Seckin, T. Pressurized liquid extraction of phenolic compounds from Anatolia propolis and their radical scavenging capacities. Food Chem. Toxicol. 2011, 49, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, S.P.; Stathopoulos, C.; Parks, S.; Roach, P. An Optimised Aqueous Extract of Phenolic Compounds from Bitter Melon with High Antioxidant Capacity. Antioxidants 2014, 3, 814-829. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox3040814
Tan SP, Stathopoulos C, Parks S, Roach P. An Optimised Aqueous Extract of Phenolic Compounds from Bitter Melon with High Antioxidant Capacity. Antioxidants. 2014; 3(4):814-829. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox3040814
Chicago/Turabian StyleTan, Sing Pei, Costas Stathopoulos, Sophie Parks, and Paul Roach. 2014. "An Optimised Aqueous Extract of Phenolic Compounds from Bitter Melon with High Antioxidant Capacity" Antioxidants 3, no. 4: 814-829. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox3040814