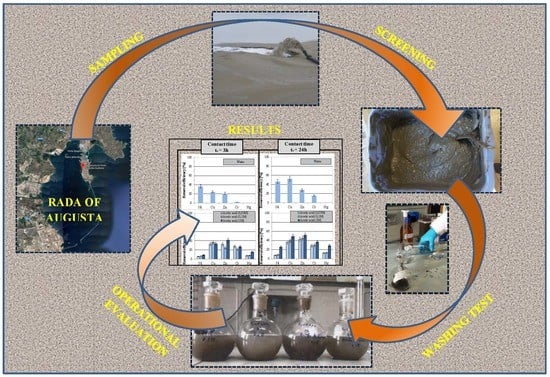
Washing Batch Test of Contaminated Sediment: The Case of Augusta Bay (SR, Italy)
Abstract
:1. Introduction
- the physical and chemical characteristics of the pollutants (hydrophobicity, water/octanol partition coefficient, solubility, oxidation state, biodegradability);
- the surface properties of the particles (ion exchange/adsorption capacity, specific surface, organic matter content, buffer capacity); and
- the chemical and physical characteristics of the portion of water in contact with the sediments (pH, Eh, alkalinity, ionic strength, salinity, temperature).
2. Materials and Methods
2.1. Sediment Sampling and Characterization
2.2. Extraction Experiments
3. Results
3.1. Sediment Characterization
- in the case of “heavy metals”, the original sediment shows contaminations that worsen critically once the sediment is pre-fractionated; and
- for almost all samples, the contamination is mainly concentrated in the fine fraction, probably due to the presence of a greater percentage of organic material on the fine fraction (see also TPH data).
- “inorganic” contamination is concentrated in the fine fraction; and
- in the case of oil pollution, the splitting operation shows a balanced distribution of TPH in both the fine and coarse matrices, with a tendency to concentrate in the fraction less than 63 micrometers.
3.2. Performance of Heavy Metal Removal with Sediment Washing
- EDTA, a widely used agent for contaminated sediment applications, which demonstrated good extraction efficiency for many heavy metals;
- citric acid, due to its high biodegradability, being a natural product, deriving from the metabolism of most living organisms;
- acetic acid, a more degradable and less toxic compound than EDTA, but having extractive and lower-cost properties; and
- deionized water, to study metal mobility. The comparison between the different selected chelating agents (Figure 2) shows that the most performing chelating agent, in terms of removal efficiency, is the citric acid. As far as the influence of concentration, it varies according to the chelating agent and the species to be complexed. In general, comparing the performances according to the concentration of the best agent (which is somehow very economical), it can be seen, already, that, at 0.05 M, the efficiencies are more than satisfactory, also on the basis of the reduction in the use of additives.
- citric acid, in general, has guaranteed for the heavy metals tendentially greater removal efficiencies, even more in an acidic environment (pH 4) and a contact time of 5 h; high removal efficiencies were obtained for Pb (80%) at 0.5 h, pH 4, and citric acid;
- in the case of the use of EDTA, the removal efficiencies of inorganic contaminants showed higher values in the basic environment (pH 9) and at the contact time of 5 h, although lower than the removal efficiencies achieved with citric acid; and
- in the case of EDDS it is the agent that showed the clearest removal efficiencies except for the Pb (~78%) at pH 9 and contact time of 5 h.
- the different forms of mercury present in the sediments interact differently with the different granulometric sediment fractions; and
- given that the methods used for the analysis on heavy metals concern a small quantity of sample (0.5 g), it is probable that the extremely variable nature of mercury (compared to other metals) is more affected by an imperfect homogenization.
3.3. TPH Removal Efficiency
3.4. Lay-Out of the “Sediment Washing” System
- maximum size of the workable sediments: 50 mm;
- material characteristics: loose (granular) non-cohesive/adhesive (plastic) material, with a maximum humidity of 25%, able to pass through a horizontal 90 mm light grid and allowing the use of a power supply unit equipped with extractor belt;
- final products: silts-clays < 0.063 mm, sand 0.063–2 mm, waste > 2 mm (gravel 4–25 mm, gravel 25–90 mm), organic material 0–90 mm, ferrous material 0–90 mm; and
- On the basis of these hypotheses and of the hypothesized general scheme, was assessed a variable treatment cost between 75 € m−3 (large scale, ≈300,000 m3) and 28 € m−3 (small scale, ≈50,000 m3); this estimates the amount of sediment eventually reused, was considered simply as a “savings” item for non-disposal, rather than a “gain” item for sale and valorization.
- “turnkey” all-inclusive plants, with the use of “economic” agents (such as citric acid) for the treatment of both organic and inorganic compounds, with a double treatment chain in parallel for the fine fraction (90%) and coarse (10%, useful for the residual mercury and hydrocarbons);
- despite all the costs were normalized by cubic meter of the treated matrix, a series of working hypotheses were put forward which provided the scale, plant and management effects, linked above all to the capital expenditure costs, validated also on the basis of literature data;
- amortization costs have provided a “constant rate of 6%” and a useful life of the plant of 15 years; and
- the evaluation of the operating expenditure costs were made on an analytical basis, with reference to the pilot and laboratory tests carried out as part of an experimental study to be carried out.
- a small-scale pilot plant, with a total cost of 1,000,000–1,500,000 euros for the treatment of around 10–15 t h−1;
- a medium–large-scale pilot plant, with a total cost of 1,750,000–2,500,000 euros for the treatment of approximately 25–30 t h−1; and
- a full-scale plant, with a total cost of 5,000,000–8,000,000 euros for treatment over 60 t h−1.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iannelli, R.; Bianchi, V.; Macci, C.; Peruzzi, E.; Chiellini, C.; Petroni, G.; Masciandaro, G. Assessment of pollution impact on biological activity and structure of seabed bacterial communities in the Port of Livorno (Italy). Sci. Total Environ. 2012, 426, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Abdel Gawad, E.A.; Al Azab, M.; Lotfy, M.M. Assessment of organic pollutants in coastal sediments, UAE. Environ. Geol. 2008, 54, 1091–1102. [Google Scholar] [CrossRef]
- Gómez-Gutiérrez, A.; Garnacho, E.; Bayona, J.M.; Albaigés, J. Assessment of the Mediterranean sediments contamination by persistent organic pollutants. Environ. Pollut. 2007, 148, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Huang, W.; Song, J.; Qian, Y.; Peng, P. Sorption of organic pollutants by marine sediments: Implication for the role of particulate organic matter. Chemosphere 2006, 65, 2493–2501. [Google Scholar] [CrossRef]
- Dastoli, S.; De Gioannis, G.; Morelli, M.; Muntoni, A.; Peretti, R.; Polettini, A.; Pomi, R.; Romano, E.; Serci, A.; Villani, B.; et al. Gestione integrata dei sedimenti provenienti dal dragaggio di piccoli porti-il progetto LIFE + “COAST-BEST”. In Proceedings of the SiCon 2014 Siti Contaminati. Esperienze Negli Interventi Di Risanamento, Brescia, Italy, 6–8 February 2014; pp. 1–12. [Google Scholar]
- Dermont, G.; Bergeron, M.; Mercier, G.; Richer-Laflèche, M. Soil washing for metal removal: A review of physical/chemical technologies and field applications. J. Hazard. Mater. 2008, 152, 1–31. [Google Scholar] [CrossRef]
- Di Palma, L.; Ferrantelli, P.; Medici, F. Heavy metals extraction from contaminated soil: Recovery of the flushing solution. J. Environ. Manag. 2005, 77, 205–211. [Google Scholar] [CrossRef]
- Di Palma, L.; Mecozzi, R. Heavy metals mobilization from harbour sediments using EDTA and citric acid as chelating agents. J. Hazard. Mater. 2007, 147, 768–775. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E.; Imborvungu, J.A. Removal of heavy metals from a contaminated soil using organic chelating acids. Int. J. Environ. Sci. Technol. 2010, 7, 485–496. [Google Scholar] [CrossRef] [Green Version]
- Andrade, M.D.; Prasher, S.O.; Hendershot, W.H. Optimizing the molarity of a EDTA washing solution for saturated-soil remediation of trace metal contaminated soils. Environ. Pollut. 2007, 147, 781–790. [Google Scholar] [CrossRef]
- Tejowulan, R.S.; Hendershot, W.H. Removal of trace metals from contaminated soils using EDTA incorporating resin trapping techniques. Environ. Pollut. 1998, 103, 135–142. [Google Scholar] [CrossRef]
- Barona, A.; Aranguiz, I.; Elías, A. Metal associations in soils before and after EDTA extractive decontamination: Implications for the effectiveness of further clean-up procedures. Environ. Pollut. 2001, 113, 79–85. [Google Scholar] [CrossRef]
- Khodadoust, A.P.; Reddy, K.R.; Maturi, K. Effect of different extraction agents on metal and organic contaminant removal from a field soil. J. Hazard. Mater. 2005, 117, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Polettini, A.; Pomi, R.; Rolle, E.; Ceremigna, D.; De Propis, L.; Gabellini, M.; Tornato, A. A kinetic study of chelant-assisted remediation of contaminated dredged sediment. J. Hazard. Mater. 2006, 137, 1458–1465. [Google Scholar] [CrossRef]
- Lee, I.H.; Wang, Y.J.; Chern, J.M. Extraction kinetics of heavy metal-containing sludge. J. Hazard. Mater. 2005, 123, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.K.A.; Li, C.; Banerji, S.K.; Regmi, T. Extraction, recovery, and biostability of EDTA for remediation of heavy metal-contaminated soil. Soil Sediment Contam. 1999, 8, 81–103. [Google Scholar] [CrossRef]
- Robles, I.; Lozano, M.J.; Solís, S.; Hernández, G.; Paz, M.V.; Olvera, M.G.; Bustos, E. Electrokinetic Treatment of Mercury-Polluted Soil Facilitated by Ethylenediaminetetraacetic Acid Coupled with A Reactor with A Permeable Reactive Barrier of Iron to Recover Mercury (II) from Water. Electrochim. Acta 2015, 181, 68–72. [Google Scholar] [CrossRef]
- Wasay, S.A.; Barrington, S.; Tokunaga, S. Organic acids for the in situ remediation of soils polluted by heavy metals: Soil flushing in columns. Water Air Soil Pollut. 2001, 127, 301–314. [Google Scholar] [CrossRef]
- Finzgar, N.; Lestan, D. The two-phase leaching of Pb, Zn and Cd contaminated soil using EDTA and electrochemical treatment of the washing solution. Chemosphere 2008, 73, 1484–1491. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, Q.; Hu, X. Extraction of Heavy Metals from Water-Stable Soil Aggregates Using EDTA. Procedia Environ. Sci. 2013, 18, 679–685. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Lee, Y.; Ong, S.K. Factors affecting EDTA extraction of lead from lead-contaminated soils. Chemosphere 2003, 51, 845–853. [Google Scholar] [CrossRef]
- Sarkar, D.; Andra, S.S.; Saminathan, S.K.M.; Datta, R. Chelant-aided enhancement of lead mobilization in residential soils. Environ. Pollut. 2008, 156, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, D.; Bermond, A.; Santos, E.; Carapuça, H.; Duarte, A. Heavy metal mobility assessment in sediments based on a kinetic approach of the EDTA extraction: Search for optimal experimental conditions. Anal. Chim. Acta 2002, 459, 245–256. [Google Scholar] [CrossRef]
- González, I.; Cortes, A.; Neaman, A.; Rubio, P. Biodegradable chelate enhances the phytoextraction of copper by Oenothera picensis grown in copper-contaminated acid soils. Chemosphere 2011, 84, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Kos, B.; Leštan, D. Chelator induced phytoextraction and in situ soil washing of Cu. Environ. Pollut. 2004, 132, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Ritschel, J. Extraction of Heavy Metals from Soil with Selected Biodegradable Complexing Agents. Master’s Thesis, University of Jena, Vergina, Germany, May 2003. [Google Scholar]
- Wang, G.; Koopmans, G.F.; Song, J.; Temminghoff, E.J.; Luo, Y.; Zhao, Q.; Japenga, J. Mobilization of heavy metals from contaminated paddy soil by EDDS, EDTA, and elemental sulfur. Environ. Geochem. Health 2007, 29, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Niinae, M.; Nishigaki, K.; Aoki, K. Removal of lead from contaminated soils with chelating agents. Mater. Trans. 2008, 49, 2377–2382. [Google Scholar] [CrossRef] [Green Version]
- Covelli, S.; Faganeli, J.; Horvat, M.; Brambati, A. Mercury contamination of coastal sediments as the result of long-term cinnabar mining activity (Gulf of Trieste, northern Adriatic sea). Appl. Geochem. 2001, 16, 541–558. [Google Scholar] [CrossRef]
- Camargo, J.A. Contribution of Spanish-American silver mines (1570–1820) to the present high mercury concentrations in the global environment: A review. Chemosphere 2002, 48, 51–57. [Google Scholar] [CrossRef]
- Orecchio, S.; Polizzotto, G. Fractionation of mercury in sediments during draining of Augusta (Italy) coastal area by modified Tessier method. Microchem. J. 2013, 110, 452–457. [Google Scholar] [CrossRef]





| Parameter | UM | Initial Unfractionated Sediment | ||||
| S1 | S2 | S3 | S4 | Composite Sample | ||
| TPH (C12-C40) | mg kgDW−1 | 30.030 | 60.600 | 220.000 | 114.020 | 177.830 |
| Ni | mg kgDW−1 | 65,905.249 | 45,080.000 | 12,540.000 | 21,813.600 | 55,834.000 |
| Cu | mg kgDW−1 | 54,821.110 | 48,840.000 | 89,740.000 | 116,331.500 | 103,235.400 |
| Zn | mg kgDW−1 | 78,173.752 | 6672.020 | 204,233.333 | 187,920.000 | 196,075.012 |
| Cr | mg kgDW−1 | 118,149.867 | 78,040.001 | 21,780.000 | 101,211.200 | 109,680.500 |
| Hg | mg kgDW−1 | 83.002 | 11.000 | 1.550 | 45.290 | 27.500 |
| Parameter | UM | Sediment Fraction < 63 µm | ||||
| S1 | S2 | S3 | S4 | Composite Sample | ||
| TPH (C12-C40) | mg kgDW−1 | 30.760 | 70.020 | 420.000 | 172.210 | 250.000 |
| Ni | mg kgDW−1 | 69,983.966 | 47,767.000 | 12,000.000 | 25,020.900 | 58,491.983 |
| Cu | mg kgDW−1 | 57,327.293 | 51,010.000 | 82,000.000 | 134,370.100 | 129,500.000 |
| Zn | mg kgDW−1 | 81,927.991 | 944.400 | 230,043.313 | 258,080.000 | 245,665.670 |
| Cr | mg kgDW−1 | 125,293.333 | 82,070.000 | 18,000.008 | 140,843.200 | 133,065.000 |
| Hg | mg kgDW−1 | 6.067 | 5.730 | 8.100 | 32.001 | 15.007 |
| Parameter | UM | Sediment Fraction < 63 Micron | ||||
| S1 | S2 | S3 | S4 | Composite Sample | ||
| TPH (C12-C40) | mg kgDW−1 | 10.000 | 10.430 | 330.000 | 40.000 | 40.230 |
| Ni | mg kgDW−1 | 19,205.000 | 31,200.000 | 15,205.080 | 15,209.800 | 17,222.700 |
| Cu | mg kgDW−1 | 25,920.400 | 3400.000 | 125,316.030 | 77,948.300 | 80,141.200 |
| Zn | mg kgDW−1 | 35,317.000 | 49,400.200 | 71,782.901 | 38,825.600 | 55,397.700 |
| Cr | mg kgDW−1 | 36,273.200 | 48,803.800 | 39,100.000 | 17,539.000 | 26,653.000 |
| Hg | mg kgDW−1 | 95.176 | 11.000 | 1.000 | 13.481 | 8.228 |
| Parameter | UM | Composite Sample | ||
|---|---|---|---|---|
| Unfractioned Sediment | <63 µm | >63 µm | ||
| TPH (C12-C40) | mg kgDW−1 | 13,567.400 | 17,840.900 | 9561.002 |
| Ni | mg kgDW−1 | 19,482.100 | 37,430.320 | 500.900 |
| Cu | mg kgDW−1 | 170.010 | 278.100 | 180.320 |
| Pb | mg kgDW−1 | 8525.020 | 14,343.510 | 850.010 |
| Cr | mg kgDW−1 | 11,090.300 | 19,411.150 | 704.310 |
| Hg | mg kgDW−1 | − | − | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lumia, L.; Giustra, M.G.; Viviani, G.; Di Bella, G. Washing Batch Test of Contaminated Sediment: The Case of Augusta Bay (SR, Italy). Appl. Sci. 2020, 10, 473. https://0-doi-org.brum.beds.ac.uk/10.3390/app10020473
Lumia L, Giustra MG, Viviani G, Di Bella G. Washing Batch Test of Contaminated Sediment: The Case of Augusta Bay (SR, Italy). Applied Sciences. 2020; 10(2):473. https://0-doi-org.brum.beds.ac.uk/10.3390/app10020473
Chicago/Turabian StyleLumia, Lucia, Maria Gabriella Giustra, Gaspare Viviani, and Gaetano Di Bella. 2020. "Washing Batch Test of Contaminated Sediment: The Case of Augusta Bay (SR, Italy)" Applied Sciences 10, no. 2: 473. https://0-doi-org.brum.beds.ac.uk/10.3390/app10020473