Finite Element Analysis for Pre-Clinical Testing of Custom-Made Knee Implants for Complex Reconstruction Surgery
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
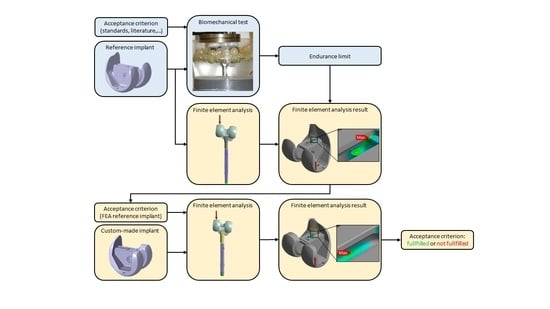
2.1. Pre-Clinical Testing Method for Custom-Made Knee Implants
2.2. Custom-Made Knee Implants
2.3. Biomechanical Test Setup
2.4. Finite Element Analysis
3. Results
3.1. Custom-Made Implant 1
3.2. Custom-Made Implant 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papagelopoulos, P.J.; Savvidou, O.D.; Koutsouradis, P.; Chloros, G.D.; Bolia, I.K.; Sakellariou, V.I.; Kontogeorgakos, V.A.; Mavrodontis, I.I.; Mavrogenis, A.F.; Diamantopoulos, P. Three-dimensional Technologies in Orthopedics. Orthopedics 2018, 41, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Camarda, L.; D’Arienzo, A.; Morello, S.; Peri, G.; Valentino, B.; D’Arienzo, M. Patient-specific instrumentation for total knee arthroplasty: A literature review. Musculoskelet. Surg. 2015, 99, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Hafez, M.A.; Moholkar, K. Patient-specific instruments: Advantages and pitfalls. SICOT J 2017, 3, 66. [Google Scholar] [CrossRef] [PubMed]
- Victor, J.; Vermue, H. Custom TKA: What to expect and where do we stand today? Arch. Orthop. Trauma Surg. 2021, 141, 2195–2203. [Google Scholar] [CrossRef]
- Levengood, G.A.; Dupee, J. Accuracy of Coronal Plane Mechanical Alignment in a Customized, Individually Made Total Knee Replacement with Patient-Specific Instrumentation. J. Knee Surg. 2018, 31, 792–796. [Google Scholar] [CrossRef] [Green Version]
- Beit Ner, E.; Dosani, S.; Biant, L.C.; Tawy, G.F. Custom Implants in TKA Provide No Substantial Benefit in Terms of Outcome Scores, Reoperation Risk, or Mean Alignment: A Systematic Review. Clin. Orthop. Relat. Res. 2021, 479, 1237–1249. [Google Scholar] [CrossRef]
- McNamara, C.A.; Gösthe, R.G.; Patel, P.D.; Sanders, K.C.; Huaman, G.; Suarez, J.C. Revision total knee arthroplasty using a custom tantalum implant in a patient following multiple failed revisions. Arthroplast. Today 2017, 3, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Burastero, G.; Pianigiani, S.; Zanvettor, C.; Cavagnaro, L.; Chiarlone, F.; Innocenti, B. Use of porous custom-made cones for meta-diaphyseal bone defects reconstruction in knee revision surgery: A clinical and biomechanical analysis. Arch. Orthop. Trauma Surg. 2020, 140, 2041–2055. [Google Scholar] [CrossRef]
- Savov, P.; Tuecking, L.-R.; Windhagen, H.; Ettinger, M. Individual Revision Knee Arthroplasty Is a Safe Limb Salvage Procedure. J. Pers. Med. 2021, 11, 572. [Google Scholar] [CrossRef]
- MacKenzie, E.J.; Jones, A.S.; Bosse, M.J.; Castillo, R.C.; Pollak, A.N.; Webb, L.X.; Swiontkowski, M.F.; Kellam, J.F.; Smith, D.G.; Sanders, R.W.; et al. Health-care costs associated with amputation or reconstruction of a limb-threatening injury. J. Bone Joint Surg. Am. 2007, 89, 1685–1692. [Google Scholar] [CrossRef]
- IMDRF. Definitions for Personalized Medical Devices: IMDRF; IMDRF: Brussels, Belgium, 2021. [Google Scholar]
- MDCG. Questions and Answers on Custom-Made Devices: MDCG 2021-3; MDCG: Brussels, Belgium, 2021. [Google Scholar]
- European Union. REGULATION (EU) 2017/745 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC: MDR 2017/745; European Union: Brussels, Belgium, 2017. [Google Scholar]
- Wehmöller, M.; Utz, M.; Gesche, V. Digital process chains for patient specific medical devices. Curr. Dir. Biomed. Eng. 2020, 6, 357–359. [Google Scholar] [CrossRef]
- Martinez-Marquez, D.; Terhaer, K.; Scheinemann, P.; Mirnajafizadeh, A.; Carty, C.P.; Stewart, R.A. Quality by Design for industry translation: Three-dimensional risk assessment failure mode, effects, and criticality analysis for additively manufactured patient-specific implants. Eng. Rep. 2020, 2, e12113. [Google Scholar] [CrossRef]
- VDI-Gesellschaft Technologies of Life Sciences. Digitale Prozessketten in der Industriellen Medizintechnik—Herstellung von Sonderanfertigungen: Digital Process Chains in Industrial Manufacturing for Medical Devices—Custom-Made Devices; (VDI 5705:2021); VDI-Gesellschaft Technologies of Life Sciences: Dusseldorf, Germany, 2021. [Google Scholar]
- Beuth. DIN EN ISO 13485:2016-08; Medizinprodukte_- Qualitätsmanagementsysteme_- Anforderungen für Regulatorische Zwecke (ISO_13485:2016); Deutsche Fassung EN_ISO_13485:2016; (DIN EN ISO 13485:2016); Beuth Verlag GmbH: Berlin, Germany, 2016. [Google Scholar]
- Taylor, M.; Prendergast, P.J. Four decades of finite element analysis of orthopaedic devices: Where are we now and what are the opportunities? J. Biomech. 2015, 48, 767–778. [Google Scholar] [CrossRef] [Green Version]
- ASME. Assessing Credibility of Computational Modeling through Verification and Validation: Application to Medical Devices: V V 40-2018; ASME: New York, NY, USA, 2018. [Google Scholar]
- Martelli, S.; Taddei, F.; Cristofolini, L.; Gill, H.S.; Viceconti, M. Extensive risk analysis of mechanical failure for an epiphyseal hip prothesis: A combined numerical-experimental approach. Proc. Inst. Mech. Eng. H 2011, 225, 126–140. [Google Scholar] [CrossRef]
- Grupp, T.M.; Kaddick, C.; Baxmann, M. Biomechanische Testung in der Orthopädie und Wirbelsäulenchirurgie. Osteologie 2015, 24, 152–157. [Google Scholar] [CrossRef]
- Cooper, H.J.; Della Valle, C.J.; Berger, R.A.; Tetreault, M.; Paprosky, W.G.; Sporer, S.M.; Jacobs, J.J. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J. Bone Joint Surg. Am. 2012, 94, 1655–1661. [Google Scholar] [CrossRef] [Green Version]
- ASTM. Standard Practice for Cyclic Fatigue Testing of Metal Tibial Tray Components of Total Knee Joint Replacements; ASTM: West Conshohocken, PA, USA, 2020. [Google Scholar]
- ISO. Chirurgische Implantate—Partieller und Totaler Hüftgelenkersatz—Teil 4: Bestimmung der Dauerwechselfestigkeit und Leistungsanforderungen an Hüftendoprothesenschäfte; ISO: London, UK, 2016. [Google Scholar]
- Bergmann, G.; Bender, A.; Graichen, F.; Dymke, J.; Rohlmann, A.; Trepczynski, A.; Heller, M.O.; Kutzner, I. Standardized loads acting in knee implants. PLoS ONE 2014, 9, e86035. [Google Scholar] [CrossRef] [Green Version]
- Grupp, T.M.; Giurea, A.; Miehlke, R.K.; Pfaff, A.; Fritz, B.; Schwiesau, J.; Blömer, W. Entwicklung einer achsgeführten Rotationsknieprothese aus biomechanischer Sicht; Design of a rotating hinge knee endoprosthesis from a biomechanical point of view. Abstr. Biomater. 2009, 47. [Google Scholar]
- Grimberg, A.; Jansson, V.; Lützner, J.; Melsheimer, O.; Morlock, M.; Steinbrück, A. German Arthroplasty Registry (Endoprothesenregister Deutschland—EPRD); EPRD: Berlin, Germany, 2020. [Google Scholar] [CrossRef]
- Vertesich, K.; Staats, K.; Böhler, C.; Koza, R.; Lass, R.; Giurea, A. Long Term Results of a Rotating Hinge Total Knee Prosthesis with Carbon-Fiber Reinforced Poly-Ether-Ether-Ketone (CFR-PEEK) as Bearing Material. Front. Bioeng. Biotechnol. 2022, 10, 845859. [Google Scholar] [CrossRef]
- Böhler, C.; Kolbitsch, P.; Schuh, R.; Lass, R.; Kubista, B.; Giurea, A. Midterm Results of a New Rotating Hinge Knee Implant: A 5-Year Follow-Up. Biomed Res. Int. 2017, 2017, 7532745. [Google Scholar] [CrossRef] [Green Version]
- Kremling, U.; Gatzke, F. Cyclic Fatigue Test on a Modified Intramedullary Adapter Stem of the Modular Tumor/Revision System “MML”. 2012. [Google Scholar]
- Favre, P.; Maquer, G.; Henderson, A.; Hertig, D.; Ciric, D.; Bischoff, J.E. In Silico Clinical Trials in the Orthopedic Device Industry: From Fantasy to Reality? Ann. Biomed. Eng. 2021, 49, 3213–3226. [Google Scholar] [CrossRef] [PubMed]
- Viceconti, M.; Pappalardo, F.; Rodriguez, B.; Horner, M.; Bischoff, J.; Musuamba Tshinanu, F. In silico trials: Verification, validation and uncertainty quantification of predictive models used in the regulatory evaluation of biomedical products. Methods 2021, 185, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Viceconti, M.; Emili, L.; Afshari, P.; Courcelles, E.; Curreli, C.; Famaey, N.; Geris, L.; Horner, M.; Jori, M.C.; Kulesza, A.; et al. Possible Contexts of Use for In Silico Trials Methodologies: A Consensus-Based Review. IEEE J. Biomed. Health Inform. 2021, 25, 3977–3982. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-G.; Park, K.-M.; Lee, H.-Y.; Park, J.-H.; Kang, K.-T. Prediction of wear performance in femoral and tibial conformity in patient-specific cruciate-retaining total knee arthroplasty. J. Orthop. Surg. Res. 2020, 15, 24. [Google Scholar] [CrossRef]
- Suh, D.-S.; Kang, K.-T.; Son, J.; Kwon, O.-R.; Baek, C.; Koh, Y.-G. Computational study on the effect of malalignment of the tibial component on the biomechanics of total knee arthroplasty: A Finite Element Analysis. Bone Joint Res. 2017, 6, 623–630. [Google Scholar] [CrossRef]
- Bitter, T.; Khan, I.; Marriott, T.; Lovelady, E.; Verdonschot, N.; Janssen, D. Finite element wear prediction using adaptive meshing at the modular taper interface of hip implants. J. Mech. Behav. Biomed. Mater. 2018, 77, 616–623. [Google Scholar] [CrossRef]
- Lange, H.-E.; Bader, R.; Kluess, D. Endurance testing and finite element simulation of a modified hip stem for integration of an energy harvesting system. Proc. Inst. Mech. Eng. H 2021, 235, 985–992. [Google Scholar] [CrossRef]
- ASTM. Standard Practice for Finite Element Analysis (FEA) of Metallic Orthopaedic Total Knee Tibial Components; ASTM: West Conshohocken, PA, USA, 2019. [Google Scholar]
- ASTM. Standard for Additive Manufacturing—Finished Part Properties—Standard Specification for Cobalt-28 Chromium-6 Molybdenum via Powder Bed Fusion; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM. Specification for Cobalt-28 Chromium-6 Molybdenum Alloy Castings and Casting Alloy for Surgical Implants (UNS R30075); ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]







| Custom-Made Implant 1 | Custom-Made Implant 2 | |||
|---|---|---|---|---|
| Clinical case and pre-operative planning |  |  |  |  |
| Implant components |  |  | ||
| Main failure mechanisms | Mechanical fatigue of proximal-anterior box area | Fretting corrosion at the taper junction | ||
| Corresponding FEA measure | Maximum tensile stress | Maximum micromotion | ||
| Post-operative image |  |  | ||
| Max. Tensile Stress [MPa] | Max. Van Mises Stress [MPa] | Max. Micromotion [mm] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Screw Tension | 5 kN | 8 kN | 10 kN | 5 kN | 8 kN | 10 kN | 5 kN | 8 kN | 10 kN |
| Reference implant | 1381 100% | 1736 100% | 2020 100% | 1734 100% | 1736 100% | 1801 100% | 0.171 100% | 0.0232 100% | 0.0197 100% |
| Custom-made implant | 509 36% | 628 36% | 725 36% | 1283 74% | 1283 74% | 1376 76% | 0.194 113% | 0.0159 68% | 0.0124 63% |
| Max. Tensile Stress [MPa] | Max. Van Mises Stress [MPa] | Max. Micromotion [mm] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Screw Tension | 5 kN | 6.5 kN | 8 kN | 5 kN | 6.5 kN | 8 kN | 5 kN | 6.5 kN | 8 kN |
| Reference implant | 551 100% | 660 100% | 772 100% | 571 100% | 637 100% | 731 100% | 0.0247 100% | 0.0237 100% | 0.0227 100% |
| Custom-made implant | 697 126% | 818 124% | 940 121% | 687 120% | 807 126% | 927 127% | 0.0225 91% | 0.0206 87% | 0.0187 82% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hettich, G.; Weiß, J.-B.; Wünsch, B.; Grupp, T.M. Finite Element Analysis for Pre-Clinical Testing of Custom-Made Knee Implants for Complex Reconstruction Surgery. Appl. Sci. 2022, 12, 4787. https://0-doi-org.brum.beds.ac.uk/10.3390/app12094787
Hettich G, Weiß J-B, Wünsch B, Grupp TM. Finite Element Analysis for Pre-Clinical Testing of Custom-Made Knee Implants for Complex Reconstruction Surgery. Applied Sciences. 2022; 12(9):4787. https://0-doi-org.brum.beds.ac.uk/10.3390/app12094787
Chicago/Turabian StyleHettich, Georg, Josef-Benedikt Weiß, Benjamin Wünsch, and Thomas M. Grupp. 2022. "Finite Element Analysis for Pre-Clinical Testing of Custom-Made Knee Implants for Complex Reconstruction Surgery" Applied Sciences 12, no. 9: 4787. https://0-doi-org.brum.beds.ac.uk/10.3390/app12094787