Identification and Characterization of Janthinobacterium svalbardensis F19, a Novel Low-C/N-Tolerant Denitrifying Bacterium
Abstract
:1. Introduction
2. Material and Methods
2.1. Media
2.2. Isolation of Strains
2.3. Bacterial Identification and Denitrification Gene Amplification
2.4. Assessment of Nitrification and Denitrification Performances
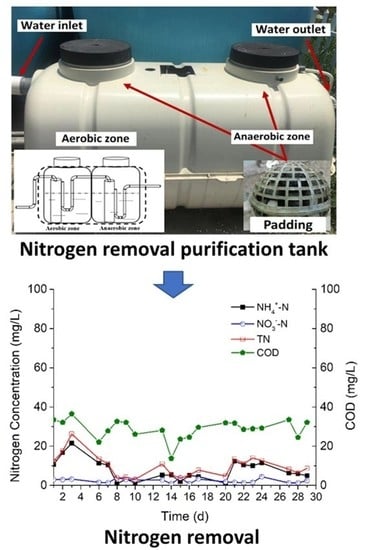
2.5. Assessment of Nitrogen Removal in Domestic Wastewater
2.6. Analytical Methods
2.7. Statistical Analysis
2.8. Nucleotide Sequence Accession Numbers
3. Results and Discussion
3.1. Isolation and Identification of F19
3.2. Factors Affecting Nitrogen Removal by Strain F19
3.2.1. Effects of C/N Ratio
3.2.2. Effects of pH and Temperature
3.2.3. Effects of Carbon Sources
3.3. Analysis of Nitrification and Denitrification Capacity
3.4. Tolerance to High-Strength Ammonium
3.5. The Potential of Strain F19 for Enhancing Nitrogen Removal from Low-C/N Domestic Sewage
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adav, S.S.; Lee, D.J.; Lai, J.Y. Enhanced biological denitrification of high concentration of nitrite with supplementary carbon source. Appl. Microbiol. Biotechnol. 2010, 85, 773–778. [Google Scholar] [CrossRef]
- Zhu, L.; Ding, W.; Feng, L.J.; Dai, X.; Xu, X.Y. Characteristics of an aerobic denitrifier that utilizes ammonium and nitrate simultaneously under the oligotrophic niche. Environ. Sci. Pollut. Res. 2012, 19, 3185–3191. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zhang, W.; Eisenhauer, N.; Liu, T.; Xiong, Y.; Liang, C.; Fu, S. Nitrogen deposition cancels out exotic earthworm effects on plant-feeding nematode communities. J. Anim. Ecol. 2017, 86, 708. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Xie, S.; Zhang, X.; Yang, Z.; Ding, W.; Liao, X.; Liu, Y.; Chen, C. Ammonium removal pathways and microbial community in GAC-sand dual media filter in drinking water treatment. J. Environ. Sci. 2012, 24, 1587–1593. [Google Scholar] [CrossRef]
- Zhou, Y.; Bai, L.; Song, C.-P. Ammonium homeostasis and signaling in plant cells. Sci. Bull. 2015, 60, 741–747. [Google Scholar] [CrossRef]
- Chen, W.; Hao, L.; Zhang, Q.; Dai, S. Effect of nitrite on growth and microcystins production of Microcystis aeruginosa PCC7806. J. Appl. Phycol. 2011, 23, 665–671. [Google Scholar] [CrossRef]
- Wałęga, A.; Chmielowski, K.; Młyński, D. Nitrogen and Phosphorus Removal from Sewage in Biofilter—Activated Sludge Combined Systems. Pol. J. Environ. Stud. 2019, 28, 1939–1947. [Google Scholar] [CrossRef]
- Wałęga, A.; Chmielowski, K.; Mły’nski, D. Influence of the Hybrid Sewage Treatment Plant’s Exploitation on Its Operation Effectiveness in Rural Areas. Sustainabilty 2018, 10, 2689. [Google Scholar] [CrossRef]
- Khin, T.; Annachhatre, A.P. Novel microbial nitrogen removal processes. Biotechnol. Adv. 2004, 22, 519–532. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Liu, Y.X.; Ren, R.P.; Lv, Y.K. Effect of temperature, salinity, heavy metals, ammonium concentration, pH and dissolved oxygen on ammonium removal by an aerobic nitrifier. RSC Adv. 2015, 5, 79988–79996. [Google Scholar] [CrossRef]
- Pal, R.R.; Khardenavis, A.A.; Purohit, H.J. Identification and monitoring of nitrification and denitrification genes in Klebsiella pneumoniae EGD-HP19-C for its ability to perform heterotrophic nitrification and aerobic denitrification. Funct. Integr. Genom. 2015, 15, 63–76. [Google Scholar] [CrossRef]
- de Almeida, R.G.B.; dos Santos, C.E.D.; Lüders, T.C.; Del Nery, V.; Leal, C.D.; Pereira, A.D.; Araújo, J.C.; Davenport, R.J.; Barana, A.C.; Lopes, D.D.; et al. Nitrogen removal by simultaneous partial nitrification, anammox and denitrification (SNAD) in a structured-bed reactor treating animal feed processing wastewater: Inhibitory effects and bacterial community. Int. Biodeterior. Biodegrad. 2018, 133, 108–115. [Google Scholar] [CrossRef]
- Feng, Q.; Yu, Y.; Guo, C.; Chen, X.; Cao, J.; Yang, W. Investigation of the influence of Ni(II) exposure on the simultaneous nitrification and denitrification of aerobic granules from an internal oxygen penetration perspective. RSC Adv. 2017, 7, 11608–11615. [Google Scholar] [CrossRef]
- Khanitchaidecha, W.; Nakaruk, A.; Koshy, P.; Futaba, K. Comparison of simultaneous nitrification and denitrification for three different reactors. Biomed. Res. Int. 2015, 2015, 1183–1190. [Google Scholar] [CrossRef]
- Jin, R.; Liu, T.; Liu, G.; Zhou, J.; Huang, J.; Wang, A. Simultaneous heterotrophic nitrification and aerobic denitrification by the marine origin bacterium Pseudomonas sp. ADN-42. Appl. Biochem. Biotechnol. 2015, 175, 2000–2011. [Google Scholar] [CrossRef]
- Miyahara, M.; Kim, S.W.; Fushinobu, S.; Takaki, K.; Yamada, T.; Watanabe, A.; Miyauchi, K.; Endo, G.; Wakagi, T.; Shoun, H. Potential of aerobic denitrification by Pseudomonas stutzeri TR2 to reduce nitrous oxide emissions from wastewater treatment plants. Appl. Environ. Microbiol. 2010, 76, 4619–4625. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Schreiber, F.; Collins, G.; Jensen, M.; Svitlica, O.; Kostka, J.; Lavik, G.; De Beer, D.; Zhou, H.; Kuypers, M. Aerobic denitrification in permeable Wadden Sea sediments. ISME J. 2011, 5, 776. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, Y.; Liu, H.; Xi, C.; Song, L. A novel heterotrophic nitrifying and aerobic denitrifying bacterium, Zobellella taiwanensis DN-7, can remove high-strength ammonium. Appl. Microbiol. Biotechnol. 2016, 100, 4219–4229. [Google Scholar] [CrossRef]
- He, T.; Li, Z.; Sun, Q.; Xu, Y.; Ye, Q. Heterotrophic nitrification and aerobic denitrification by Pseudomonas tolaasii Y-11 without nitrite accumulation during nitrogen conversion. Bioresour. Technol. 2016, 200, 493–499. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, R.; Zhang, Z.; Wang, X.; Ye, X.; Chen, S. Synergy of carbon and nitrogen removal of a co-culture of two aerobic denitrifying bacterial strains, Acinetobacter sp. GA and Pseudomonas sp. GP. RSC Adv. 2018, 8, 21558–21565. [Google Scholar] [CrossRef]
- Su, J.F.; Shao, S.C.; Huang, T.L.; Ma, F.; Zhang, K.; Wen, G.; Zheng, S.C. Isolation, identification, and algicidal activity of aerobic denitrifying bacterium R11 and its effect on Microcystis aeruginosa. Water Sci. Technol. 2016, 73, 2600–2607. [Google Scholar] [CrossRef]
- Padhi, S.K.; Tripathy, S.; Sen, R.; Mahapatra, A.S.; Mohanty, S.; Maiti, N.K. Characterization of heterotrophic nitrifying and aerobic denitrifying Klebsiella pneumoniae CF-S9 strain for bioremediation of wastewater. Int. Biodeterior. Biodegrad. 2013, 78, 67–73. [Google Scholar] [CrossRef]
- Kong, Q.X.; Wang, X.W.; Jin, M.; Shen, Z.Q.; Li, J.W. Development and application of a novel and effective screening method for aerobic denitrifying bacteria. FEMS Microbiol. Lett. 2006, 260, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Jin, P.; Chen, Y.; Zheng, Z.; Du, Q. Evaluation of a novel low-carbon to nitrogen- and temperature-tolerant simultaneously nitrifying–denitrifying bacterium and its use in the treatment of river water. RSC Adv. 2018, 8, 27417–27422. [Google Scholar] [CrossRef]
- Duan, J.; Fang, H.; Su, B.; Chen, J.; Lin, J. Characterization of a halophilic heterotrophic nitrification-aerobic denitrification bacterium and its application on treatment of saline wastewater. Bioresour. Technol. 2015, 179, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, J.; Li, Q.X.; Wang, Y.; Li, S.; Ren, T.; Wang, L. Simultaneous heterotrophic nitrification and aerobic denitrification by bacterium Rhodococcus sp. CPZ24. Bioresour. Technol. 2012, 116, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Lu, D.; Qin, B.; Liu, Q.; Zhao, Y.; Liu, H.; Ma, J. Highly efficient nitrogen removal of a coldness-resistant and low nutrient needed bacterium, Janthinobacterium sp. M-11. Bioresour. Technol. 2018, 256, 366–373. [Google Scholar]
- Chen, Q.; Ni, J. Ammonium removal by Agrobacterium sp. LAD9 capable of heterotrophic nitrification–aerobic denitrification. J. Biosci. Bioeng. 2012, 113, 619–623. [Google Scholar]
- Liu, Y.; Wang, Y.; Li, Y.; An, H.; Lv, Y. Nitrogen removal characteristics of heterotrophic nitrification-aerobic denitrification by C16. Chin. J. Chem. Eng. 2015, 23, 827–834. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Liu, Y.; Ai, G.M.; Miao, L.L.; Zheng, H.Y.; Liu, Z.P. The characteristics of a novel heterotrophic nitrification-aerobic denitrification bacterium, Bacillus methylotrophicus strain L7. Bioresour. Technol. 2012, 108, 35–44. [Google Scholar] [CrossRef]
- Huang, F.; Pan, L.; Lv, N.; Tang, X. Characterization of novel Bacillus strain N31 from mariculture water capable of halophilic heterotrophic nitrification-aerobic denitrification. J. Biosci. Bioeng. 2017, 124, 564–571. [Google Scholar] [CrossRef]
- Singh, A.; Agrawal, M.; Marshall, F.M. The role of organic vs. inorganic fertilizers in reducing phytoavailability of heavy metals in a wastewater-irrigated area. Ecol. Eng. 2010, 36, 1733–1740. [Google Scholar] [CrossRef]
- Lebeau, T.; Braud, A.; Jézéquel, K. Performance of bioaugmentation-assisted phytoextraction applied to metal contaminated soils: A review. Environ. Pollut. 2008, 153, 497–522. [Google Scholar] [CrossRef]
- Samorì, G.; Samorì, C.; Guerrini, F.; Pistocchi, R. Growth and nitrogen removal capacity of Desmodesmus communis and of a natural microalgae consortium in a batch culture system in view of urban wastewater treatment: Part I. Water Res. 2013, 47, 791–801. [Google Scholar] [CrossRef]
- Qu, D.; Wang, C.; Wang, Y.; Zhou, R.; Ren, H. Heterotrophic nitrification and aerobic denitrification by a novel groundwater origin cold-adapted bacterium at low temperatures. RSC Adv. 2014, 5, 5149–5157. [Google Scholar] [CrossRef]
- Takaya, N.; Catalan-Sakairi, M.A.B.; Sakaguchi, Y.; Kato, I.; Zhou, Z.; Shoun, H. Aerobic denitrifying bacteria that produce low levels of nitrous oxide. Appl. Environ. Microbiol. 2003, 69, 3152–3157. [Google Scholar] [CrossRef]
- Meng, L.; Tim, F.; Xiaoyan, L.; Ji-Dong, G. Cytochrome cd1-containing nitrite reductase encoding gene nirS as a new functional biomarker for detection of anaerobic ammonium oxidizing (Anammox) bacteria. Environ. Sci. Technol. 2011, 45, 3547–3553. [Google Scholar]
- Ren, Y.X.; Yang, L.; Liang, X. The characteristics of a novel heterotrophic nitrifying and aerobic denitrifying bacterium, Acinetobacter junii YB. Bioresour. Technol. 2014, 171, 1–9. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Jin, P.; Cui, Z.; Xu, T.; Zhao, R.; Zheng, Z. Identification and Characterization of Janthinobacterium svalbardensis F19, a Novel Low-C/N-Tolerant Denitrifying Bacterium. Appl. Sci. 2019, 9, 1937. https://0-doi-org.brum.beds.ac.uk/10.3390/app9091937
Chen Y, Jin P, Cui Z, Xu T, Zhao R, Zheng Z. Identification and Characterization of Janthinobacterium svalbardensis F19, a Novel Low-C/N-Tolerant Denitrifying Bacterium. Applied Sciences. 2019; 9(9):1937. https://0-doi-org.brum.beds.ac.uk/10.3390/app9091937
Chicago/Turabian StyleChen, Yinyan, Peng Jin, Zhiwen Cui, Tao Xu, Ruojin Zhao, and Zhanwang Zheng. 2019. "Identification and Characterization of Janthinobacterium svalbardensis F19, a Novel Low-C/N-Tolerant Denitrifying Bacterium" Applied Sciences 9, no. 9: 1937. https://0-doi-org.brum.beds.ac.uk/10.3390/app9091937