Review of Radiation-Induced Effects in Polyimide
Abstract
:1. Introduction
2. GEO-Environment Simulation Facilities
3. Results
3.1. Changes in Electrical Properties
3.2. Changes in Chemical Properties
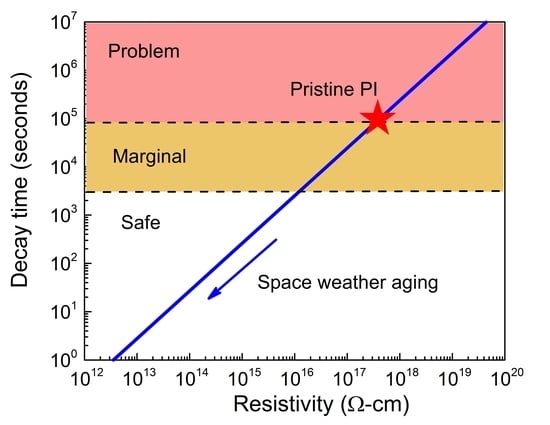
3.3. Change in Dielectric Properties
3.4. Changes in Mechanical Properties
3.5. Changes in Physical Properties
3.5.1. Optical
3.5.2. Surface Morphology
3.6. Other Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sroog, C.E.; Endrey, A.L.; Abramo, S.V.; Berr, C.E.; Edwards, W.M.; Olivier, K.L. Aromatic polypyromellitimides from aromatic polyamic acids. J. Polym. Sci. 1965, 3, 1373–1390. [Google Scholar] [CrossRef]
- Iyer, K.A.; Mehoke, D.S.; Batra, R.C. Interplanetary Dust Particle Shielding Capability of Spacecraft Multilayer Insulation. J. Spacecr. Rocket. 2015, 52, 584–594. [Google Scholar] [CrossRef]
- Christiansen, E.L.; Lear, D.M. Toughened Thermal Blanket for Micrometeoroid and Orbital Debris Protection. Procedia Eng. 2015, 103, 73–80. [Google Scholar] [CrossRef]
- Mazzinghi, A.; Sabbadini, M.; Freni, A. Enhanced RF Behavior Multi-Layer Thermal Insulation. Sci. Rep. 2018, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Hołyńska, M.; Tighe, A.; Semprimoschnig, C. Coatings and Thin Films for Spacecraft Thermo-Optical and Related Functional Applications. Adv. Mater. Intterfaces 2018, 5, 1701644. [Google Scholar] [CrossRef]
- Berger, M.J.; Coursey, J.S.; Zucker, M.A.; Chang, J. ESTAR, PSTAR, and ASTAR: Computer Programs for Calculating Stopping-Power and Range Tables for Electrons, Protons, and Helium Ions (Version 1.2.1); National Institute of Standards and Technology: Gaithersburg, MD, USA, 1999. Available online: http://physics.nist.gov/Star (accessed on 11 November 2018).
- Jursa, A.S.; Laboratory USAFG. Handbook of Geophysics and the Space Environment; Air Force Geophysics Laboratory, Air Force Systems Command, U.S. Air Force: Baltimore, Maryland, 1985.
- Ginet, G.; O’Brien, T.; Huston, S.; Johnston, W.; Guild, T.; Friedel, R.; Lindstrom, C.D.; Roth, C.J.; Whelan, P.; Quinn, R.A.; et al. AE9, AP9 and SPM: New models for specifying the trapped energetic particle and space plasma environment. Space Sci. Rev. 2013, 179, 579–615. [Google Scholar] [CrossRef]
- Paillous, A. Radiation damage to surface and structure materials. In The Behavior of Systems in the Space Environment; Springer: Dordrecht, The Netherlands, 1993; pp. 383–405. [Google Scholar]
- Pilipenko, V.; Yagova, N.; Romanova, N.; Allen, J. Statistical relationships between satellite anomalies at geostationary orbit and high-energy particles. Adv. Space Res. 2006, 37, 1192–1205. [Google Scholar] [CrossRef]
- Schühle, U.; Halain, J.-P.; Meining, S.; Teriaca, L. The Lyman-alpha telescope of the extreme ultraviolet imager on solar orbiter. Proc. SPIE 2011, 8148, 81480K-1–81480K-11. [Google Scholar] [CrossRef]
- Sato, N.; Suzuki, K.; Chiba, U.; Miyake, H.; Tanaka, Y.; Okumura, T.; Kawakita, S.; Takahashi, M.; Koga, K. Photoelectron Emission on Polyimide Films Irradiated by Proton. In Proceedings of the IEEE 2018 Condition Monitoring and Diagnosis (CMD), Perth, WA, Australia, 23–26 September 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Arnaout, M.; Paulmier, T.; Dirassen, B.; Payan, D. Study of radiation induced conductivity and photoconduction phenomenon for materials used in space environment. J. Electrost. 2016, 84, 48–53. [Google Scholar] [CrossRef]
- Wu, J.; Miyahara, A.; Khan, A.; Iwata, M.; Toyoda, K.; Cho, M.; Zheng, X. Effects of energetic electron and proton irradiation on electron emission yield of polyimide induced by electron and photon. Trans. Jpn. Soc. Aeronaut. Space Sci. 2014, 12, 13–19. [Google Scholar] [CrossRef]
- Marletta, G.; Iacona, F. Heat-induced versus particle-beam-induced chemistry in polyimide. Nucl. Instrum. Methods Phys. Res. B 1993, 80, 1045–1049. [Google Scholar] [CrossRef]
- Qu, C.; Hu, J.; Liu, X.; Li, Z.; Ding, Y. Morphology and Mechanical Properties of Polyimide Films: The Effects of UV Irradiation on Microscale Surface. Materials 2017, 10, 1329. [Google Scholar] [CrossRef]
- Marletta, G.; Iacona, F. Chemical and Physical Property Modifications Induced by Ion Irradiation in Polymers. In Materials and Processes for Surface and Interface Engineering. NATO ASI Series (Series E: Applied Sciences); Pauleau, Y., Ed.; Springer: Dordrecht, The Netherlands, 1995; Volume 290. [Google Scholar] [CrossRef]
- Dennison, J.R.; Sim, A.; Thomson, C. Evolution of the Electron Yield Curves of Insulators as a Function of Impinging Electron Fluence and Energy. IEEE Trans. Plasma Sci. 2006, 34, 2204–2218. [Google Scholar] [CrossRef] [Green Version]
- Wilson, G.; Dennison, J.R. Approximation of range in materials as a function of incident electron energy. IEEE Trans. Plasma Sci. 2012, 40, 291–297. [Google Scholar] [CrossRef]
- Dennison, J.R.; Lundgreen, P.; Christensen, J. Parameterization of Secondary and Backscattered Electron Yields for Spacecraft Charging. 2018. Available online: https://digitalcommons.usu.edu/mp_post/74 (accessed on 14 June 2018).
- Marletta, G. Chemical reactions and physical property modifications induced by keV ion beams in polymers. Nucl. Instrum. Methods Phys. Res. B 1990, 46, 295–305. [Google Scholar] [CrossRef]
- O’Brien, T.P.; Johnston, W.R.; Huston, S.L.; Roth, C.J.; Guild, T.B.; Su, Y.J.; Quinn, R.A. Changes in AE9/AP9-IRENE Version 1.5. IEEE Trans. Nucl. Sci. 2018, 65, 462–466. [Google Scholar] [CrossRef]
- Space Weather Prediction Center: US National Oceanographic and Atmospheric Administration. 2018. Available online: https://www.swpc.noaa.gov/products/ace-real-time-solar-wind (accessed on 5 June 2018).
- Xapsos, M.A.; Stauffer, C.; Gee, G.B.; Barth, J.L.; Stassinopoulos, E.G.; McGuire, R.E. Model for solar proton risk assessment. IEEE Trans. Nucl. Sci. 2004, 51, 3394–3398. [Google Scholar] [CrossRef] [Green Version]
- Tyssey, H.N.; Stadsnes, J. Cutoff latitude variation during solar proton events: Causes and consequences. J. Geophys. Res. Space Phys. 2015, 120, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Wall, J.A.; Burke, E.A.; Frederickson, A.R. Results of Literature Search on Dielectric Properties and Electron. Interaction Phenomena Related to Spacecraft Charging; NASA Report; NASA: Washington, DC, USA, 1977.
- Rose, M.F. Electrical insulation and dielectrics in the space environment. IEEE Trans. Electr. Insul. 1987, 5, 555. [Google Scholar] [CrossRef]
- Laghari, J.R.; Hammoud, A.N. A brief survey of radiation effects on polymer dielectrics. IEEE Trans. Nucl. Sci. 1990, 37, 1076. [Google Scholar] [CrossRef]
- Plis, E.; Engelhart, D.P.; Barton, D.; Cooper, R.; Ferguson, D.; Hoffmann, R. Degradation of polyimide under exposure to 90 keV electrons. Phys. Status Solidi B 2017, 254, 1600819. [Google Scholar] [CrossRef]
- Paulmier, T.; Dirassen, B.; Arnaout, M.; Payan, D.; Balcon, N. Radiation-Induced Conductivity of Space Used Polymers Under High Energy Electron Irradiation. IEEE Trans. Plasma Sci. 2015, 43, 2907–2914. [Google Scholar] [CrossRef]
- Stiegman, A.E.; Liang, R.H. Ultraviolet and vacuum-ultraviolet radiation effects on spacecraft thermal control materials. In The Behavior of Systems in the Space Environment; Springer: Dordrecht, The Netherlands, 1993; pp. 259–266. [Google Scholar]
- Kan, H.K.A. Space environment effects on spacecraft surface materials. Proc. SPIE 1985, 541, 164–180. [Google Scholar] [CrossRef]
- Lippert, T. Interaction of photons with polymers: From surface modification to ablation. Plasma Process. Polym. 2005, 2, 525–546. [Google Scholar] [CrossRef]
- Plis, E.; Engelhart, D.P.; Cooper, R.; Ferguson, D.C.; Hoffmann, R. Effect of environment on charge transport properties of polyimide films damaged by high-energy electron radiation. J. Vac. Sci. Technol. B 2018, 36, 52906. [Google Scholar] [CrossRef]
- Galabova, K.K.; de Weck, O.L. Economic case for the retirement of geosynchronous communication satellites via space tugs. Acta Astronaut. 2006, 58, 485–498. [Google Scholar] [CrossRef]
- Du, X.; Liang, B.; Xu, W.; Qiu, Y. Pose measurement of large non-cooperative satellite based on collaborative cameras. Acta Astronaut. 2011, 68, 2047–2065. [Google Scholar] [CrossRef]
- Kaiser, C.; Sjöberg, F.; Delcura, J.M.; Eilertsen, B. SMART-OLEV—An orbital life extension vehicle for servicing commercial spacecrafts in GEO. Acta Astronaut. 2008, 63, 400–410. [Google Scholar] [CrossRef]
- Chen, X.Q.; Yu, J. Optimal mission planning of GEO on-orbit refueling in mixed strategy. Acta Astronaut. 2017, 133, 63–72. [Google Scholar] [CrossRef]
- Han, C.; Zhang, S.; Wang, X. On-orbit servicing of geosynchronous satellites based on low-thrust transfers considering perturbations. Acta Astronaut. 2019. [Google Scholar] [CrossRef]
- Mateo-Velez, J.-C.; Sicard-Piet, A.; Lazaro, D.; Inguimbert, V.; Sarrailh, P.; Hess, S.; Maget, V.; Payan, D. Severe Geostationary Environments: Numerical Estimation of Spacecraft Surface Charging from Flight Data. J. Spacecr. Rocket. 2016, 53, 304–316. [Google Scholar] [CrossRef]
- Iucci, N.; Levitin, A.E.; Belov, A.V.; Eroshenko, E.A.; Ptitsyna, N.G.; Villoresi, G.; Chizhenkov, G.V.; Dorman, L.I.; Gromova, L.I.; Parisi, M.; et al. Space weather conditions and spacecraft anomalies in different orbits. Space Weather 2005, 3, S01001–S01016. [Google Scholar] [CrossRef]
- Renger, T.; Sznajder, M.; Witzke, A.; Geppert, U. The Complex Irradiation Facility at DLR-Bremen. In Advances in Solar Sailing; Macdonald, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Cooper, R.; Hoffmann, R. Jumbo Space Environment Simulation and Spacecraft Charging Chamber Characterization; Air Force Report No. AFRL-RV-PS-TP-2015-0012; Air Force Research Laboratory: Albuquerque, NM, USA, 2015. [Google Scholar]
- Test Facility Guide. Available online: https://www.arnold.af.mil/Portals/49/documents/AFD-080625-010.pdf?ver=2016-06-16-100801-260 (accessed on 1 July 2018).
- Boeing Radiation Effect Laboratory. Available online: http://www.boeing.com/specialty/radiation-effects-laboratory/index.page (accessed on 7 July 2018).
- Dirassen, B.; Levy, L.; Reulet, R.; Payan, D. ; Fletcher, K. (Compiler) ESA SP-540. The SIRENE facility—An improved method for simulating the charge of dielectrics in a charging electron environment. In Proceedings of the 9th International Symposium on Materials in a Space Environment, Noordwijk, The Netherlands, 16–20 June 2003; ESA Publications Division: Noordwijk, Netherlands, 2003; pp. 351–358, ISBN 92-9092-850-6. [Google Scholar]
- National Institutes for Quantum and Radiological Science and Technology. Available online: http://www.taka.qst.go.jp/tiara/665/english/Acc_index.php (accessed on 10 July 2018).
- Bajpai, P.K.; Trivedi, T.; Patel, S.P.; Mallik, C.; Chaturvedi, L. National Centre for Accelerator based Research at GGV Bilaspur: Emerging facility for Neutron Generation. Proc. Dae Symp. Nucl. Phys. 2013, 58, 962–963. Available online: www.sympnp.org/proceedings (accessed on 15 July 2018).
- Taiwan Daheng Irradiation Plant. Available online: http://www.dasheng.com/en/taiwan.html (accessed on 15 July 2018).
- Irradiation Facilities Database. Available online: http://irradiation-facilities.web.cern.ch/ (accessed on 15 July 2018).
- Hoffmann, R.; Dennison, J.R.; Thomson, C.D.; Albretsen, J. Low-fluence Electron Yields of Highly Insulating Materials. IEEE Trans. Plasma Sci. 2008, 36, 2238–2245. [Google Scholar] [CrossRef]
- Frederickson, A.R.; Dennison, J.R. Measurement of conductivity and charge storage in insulators related to spacecraft charging. IEEE Trans. Nucl. Sci. 2003, 50, 2284–2291. [Google Scholar] [CrossRef]
- Plis, E.A.; Engelhart, D.P.; Likar, J.; Hoffmann, R.C.; Cooper, R.; Ferguson, D. Electrical behavior of carbon-loaded Kapton for spacecraft applications. J. Spacecr. Rocket. 2017, 55, 775–777. [Google Scholar] [CrossRef]
- ASTM. Standard Test. Methods for DC Resistance or Conductance of Insulating Materials; ASTM: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Dennison, J.R.; Brunson, J.; Swaminathan, P.; Green, N.W.; Frederickson, A.R. Methods for High Resistivity Measurements Related to Spacecraft-Charging. IEEE Trans. Plasma Sci. 2006, 34, 2191–2203. [Google Scholar] [CrossRef] [Green Version]
- Engelhart, D.P.; Plis, E.A.; Cooper, R.; Ferguson, D.; Johnston, W.R.; Hoffmann, R.C. Space Plasma Interactions with Spacecraft Materials. In Plasma Science and Technology—Basic Fundamentals and Modern Applications; InTechOpen Limited: London, UK, 2018. [Google Scholar] [CrossRef]
- Frederickson, A.R.; Benson, C.E.; Bockman, J.F. Measurement of charge storage and leakage in polyimides. Nucl. Instrum. Methods Phys. Res. B 2003, 208, 454–460. [Google Scholar] [CrossRef]
- Paulmer, T.; Dirassen, B.; Payan, D.; van Eesbeek, M. Material charging in space environment: Experimental test simulation and induced conductive mechanisms. IEEE Trans. Dielectr. Elect. Insul. 2009, 16, 682–688. [Google Scholar] [CrossRef]
- Motyl, E. Electrode effects and electrical conduction in polyimide Kapton HN films. In Proceedings of the IEEE Proceedings of 6th International Conference on Conduction and Breakdown in Solid Dielectrics, Vasteras, Sweden, 22–25 June 1998; pp. 237–240. [Google Scholar] [CrossRef]
- Perrin, C.; Griseri, V.; Inguimbert, C.; Laurent, C. Analysis of internal charge distribution in electron irradiated polyethylene and polyimide films using a new experimental method. J. Phys. D Appl. Phys. 2008, 41, 205417. [Google Scholar] [CrossRef]
- Tanaka, Y.; Fukuyoshi, F.; Osawa, N.; Miyake, H.; Takada, T.; Watanabe, R.; Tomita, N. Acoustic measurement techniques for bulk charge distribution in dielectrics irradiated by electron beam. In Proceedings of the IEEE Proceedings of International Conference on Solid Dielectrics, Toulouse, France, 5–9 July 2004; Volume 2, pp. 940–943. [Google Scholar] [CrossRef]
- Mo, Y.; Zhang, L.; Zhou, Y.; Liu, J.; Zhang, Y.; Zhou, Z. Space charge characteristics of thermally and electrically aged polyimide films. In Proceedings of the 12th International Conference on the Properties and Applications of Dielectric Materials (ICPADM), Xi’an, China, 20–24 May 2018; pp. 1053–1056. [Google Scholar] [CrossRef]
- Dennison, J.; Gillespie, J.; Hodges, J.; Hoffmann, R.; Abbott, J.; Hunt, A.W. Radiation Induced Conductivity of Highly-Insulating Spacecraft Materials. In Proceedings of the 10th Spacecraft Charging Technology Conference, Biarritz, France, 18–21 June 2007. [Google Scholar]
- Blaise, G. Charge localization and transport in disordered dielectric materials. J. Electrostat. 2001, 50, 69–89. [Google Scholar] [CrossRef]
- Sessler, G.M. Charge dynamics in irradiated polymers. IEEE Trans. Electr. Insul. 1992, 27, 23–42. [Google Scholar] [CrossRef]
- Sessler, G.M.; Yang, G.M. Charge dynamics in electron-irradiated polymers. Braz. J. Phys. 1999, 29, 233–240. [Google Scholar] [CrossRef]
- Cao, M.; Wang, F.; Liu, J.; Zhang, H.-B. Charging dynamics of a polymer due toelectron irradiation: A simultaneous scattering-transport model and preliminary results. Chin. Phys. B 2012, 21, 127901. [Google Scholar] [CrossRef]
- Feng, G.B.; Cao, M.; Yan, L.P.; Zhang, H.B. Combined effects of sample parameters on polymer charging due to electron irradiation: A contour simulation. Micron 2013, 52–53, 62–66. [Google Scholar] [CrossRef]
- Li, W.Q.; Zhang, H.B. The surface potential of insulating thin films negatively charged by a low-energy focused electron beam. Micron 2010, 41, 416–422. [Google Scholar] [CrossRef]
- Molinie, P. A review of mechanisms and models accounting for surface potential decay. IEEE Trans. Plasma Sci. 2012, 40, 167–176. [Google Scholar] [CrossRef]
- Tyutnev, A.; Saenko, V.; Pozhidaev, E.; Ikhsanov, R. Experimental and theoretical studies of radiation-induced conductivity in spacecraft polymers. IEEE Trans. Plasma Sci. 2015, 43, 2915–2924. [Google Scholar] [CrossRef]
- Sessler, G.M. Charge distribution and transport in polymers. IEEE Trans. Dielectr. Electr. Insul. 1997, 4, 614–628. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.B. Analysis of microscopic parameters of surface charging in polymer caused by defocused electron beam irradiation. Micron 2014, 67, 74–80. [Google Scholar] [CrossRef]
- Yang, G.M.; Sessler, G.M. Radiation-induced conductivity in electron-beam irradiated insulating polymer films. IEEE Trans. Electr. Insul. 1992, 27, 843–848. [Google Scholar] [CrossRef]
- Gross, B.; Faria, R.M.; Ferreira, G.F. Radiation induced conductivity in Teflon irradiated by X-rays. J. Appl. Phys. 1981, 52, 571–578. [Google Scholar] [CrossRef]
- Tyutnev, A.P.; Saenko, V.S.; Smirnov, I.A.; Pozhidaev, E.D. Radiation-induced conductivity in polymers during long-term irradiation. High Energy Chem. 2006, 40, 319–330. [Google Scholar] [CrossRef]
- Hanna, R.; Paulmier, T.; Molinie, P.; Belhaj, M.; Dirassen, B.; Payan, D.; Balcon, N. Radiation induced conductivity in space dielectric materials. J. Appl. Phys. 2014, 115, 33713. [Google Scholar] [CrossRef]
- Paulmier, T.; Dirassen, B.; Rey, R. Electrostatic behaviour of space used materials in regard of internal charging met on spacecrafts. J. Electrostat. 2018, 92, 66–74. [Google Scholar] [CrossRef]
- Treadaway, M.J.; Mallon, C.E.; Flanagan, T.M.; Denson, R.; Wenaas, E.P. The effects of high-energy electrons on the charging of spacecraft dielectrics. IEEE Trans. Nucl. Sci. 1979, 26, 5101–5106. [Google Scholar] [CrossRef]
- Hoffmann, R.; Cooper, R.; Ferguson, D. Changes of the Electrical and Optical Character of Polyimide Films Due to Exposure to High Energy GEO-like Electrons and the Chemistry that Drives it. In Proceedings of the Advanced Maui Optical and Space Surveillance Technologies Conference, Maui, HI, USA, 15–18 September 2014. [Google Scholar]
- Gartstein, Y.N.; Conwell, E.M. Off-diagonal disorder and activation-energy of high-field hopping motion. Phys. Rev. B 1995, 51, 6947–6952. [Google Scholar] [CrossRef]
- Levy, L.; Paulmier, T.; Dirassen, B.; Inguimbert, C.; van Eesbeek, M. Aging and Prompt Effects on Space Material Properties. IEEE Trans. Plasma Sci. 2008, 36, 2228–2237. [Google Scholar] [CrossRef]
- Molinie, P.; Dessante, P.; Hanna, R.; Paulmier, T.; Dirassen, B.; Belhaj, M.; Payan, D.; Balcon, N. Polyimide and FEP charging behavior under multienergetic electron-beam irradiation. IEEE Trans. Dielect. Electr. Insul. 2012, 19, 1215–1220. [Google Scholar] [CrossRef]
- Paulmier, T.; Hanna, R.; Belhaj, M.; Dirassen, B.; Payan, D.; Balcon, N.; Tonon, C.; Dantras, E.; Bernès, A. Aging Effect and Induced Electric Phenomena on Dielectric Materials Irradiated with High Energy Electrons. IEEE Trans. Plasma Sci. 2013, 41, 3422–3427. [Google Scholar] [CrossRef]
- Paulmier, T.; Dirassen, B.; Payan, D.; Arnaout, M. Analysis of charge transport and ionization effect in space-used polymers under high-energy electron irradiation. IEEE Trans. Plasma Sci. 2017, 45, 1933–1937. [Google Scholar] [CrossRef]
- Yue, L.; Wu, Y.; Sun, C.; Shi, Y.; Zhang, Y. Effects of proton pre-irradiation on radiation induced conductivity of polyimide. Rad. Phys. Chem. 2016, 119, 130–135. [Google Scholar] [CrossRef]
- Yue, L.; Wang, X.; Wu, Y.; Cao, J.L.; Liu, Y.; Sun, C.; Yang, J. Study on evolution of deep charge traps in polyimide irradiated by low-energy protons using photo-stimulated discharge technique. J. Phys. D Appl. Phys. 2013, 46, 145502. [Google Scholar] [CrossRef]
- Hude, V.; Robben, A.P. Investigation of the effect of proton irradiation on the insulation properties of Kapton. In Proceedings of the ESA, European Space Power Conference, Tarragona, Spain, 21–25 September 1998; Volume 2, pp. 627–631. [Google Scholar]
- Schumann, M.; Sauerbrey, R.; Smayling, M.C. Permanent increase of the electrical conductivity of polymers induced by ultraviolet laser radiation. Appl. Phys. Lett. 1991, 58, 428–430. [Google Scholar] [CrossRef]
- Feurer, T.; Sauerbrey, R.; Smayling, M.C.; Story, B.J. Ultraviolet-Laser-Induced Permanent Electrical Conductivity in Polyimide. Appl. Phys. A 1993, 56, 275–281. [Google Scholar] [CrossRef]
- Srinivasan, R.; Hall, R.R.; Wilson, W.D.; Loehle, W.D.; Allbee, D.C. Ultraviolet laser irradiation of the polyimide, PMDA-ODA (Kapton™), to yield a patternable, porous, electrically conducting carbon network. Synth. Met. 1994, 66, 301–307. [Google Scholar] [CrossRef]
- Nurmukhametov, R.; Likhachev, D.Y.; Lavrov, S.; Kardash, J.Y. Features of electronic absorption spectra of aromatic polyimides and polyisoimides. Polym. Sci. USSR 1989, 31, 434–440. [Google Scholar] [CrossRef]
- Ortelli, E.; Geiger, F.; Lippert, T.; Wokaun, A. Pyrolysis of Kapton® in air: An in situ DRIFT study. Appl. Spectrosc. 2001, 55, 412–419. [Google Scholar] [CrossRef]
- Steckenreiter, T.; Balanzat, E.; Fuess, H.; Trautmann, C. Pyrolytic effects induced by energetic ions in polymers. Nucl. Instrum. Meth. B 1999, 151, 161–168. [Google Scholar] [CrossRef]
- Ha, K.; West, J.L. Studies on the photodegradation of polarized UV-exposed PMDA–ODA polyimide films. J. Appl. Polym. Sci. 2002, 86, 3072–3077. [Google Scholar] [CrossRef]
- Engelhart, D.P.; Plis, E.; Humagain, S.; Greenbaum, S.; Ferguson, D.; Cooper, R.; Hoffmann, R. Chemical and Electrical Dynamics of Polyimide Film Damaged by Electron Radiation. IEEE Trans. Plasma Sci. 2017, 45, 2573–2577. [Google Scholar] [CrossRef]
- Guenther, M.; Gerlach, G.; Suchaneck, G.; Sahre, K.; Eichhorn, K.-J.; Wolf, D.B.; Deineka, A.; Jastrabik, L. Ion-Beam Induced Chemical and Structural Modification in Polymers. Surf. Coat. Technol. 2002, 158–159, 108–113. [Google Scholar] [CrossRef]
- Atta, A. Modification of the Surface Properties of Polyimide Films Using Oxygen Plasma Exposure. Arab J. Nucl. Sci. Appl. 2013, 46, 115–123. [Google Scholar]
- Shrinet, V.; Chaturvedi, U.K.; Agrawal, S.K.; Rai, V.N.; Nigam, A.K. Effect of Neutron and Proton Irradiation on Some Properties of Kapton. In Polyimides; Mittal, K.L., Ed.; Springer: Boston, MA, USA, 1984. [Google Scholar] [CrossRef]
- Humagain, S.; Jhonson, J.; Stallworth, P.; Engelhart, D.; Plis, E.; Ferguson, D.; Cooper, R.; Hoffmann, R.; Greenbaum, S. Study of Damage and Recovery of Electron Irradiated Polyimide using EPR and NMR Spectroscopy. In Proceedings of the APS Meeting, New Orleans, Louisiana, 13–17 March 2017. [Google Scholar]
- Havens, J.R.; Ishida, H.; Koenig, J.L. 1. High-resolution carbon-13 nuclear magnetic resonance study of conjugation in solid polyimides. Macromolecules 1981, 14, 1327–1333. [Google Scholar] [CrossRef]
- O’Donnell, J.H.; Whittaker, A.K. The high resolution 13C NMR spectrum of poly(N,N′-bis(phenoxyphenyl) pyromellitimide), (Kapton). Polym. Bull. 1984, 12, 319. [Google Scholar] [CrossRef]
- Long, E.R., Jr.; Long, S.A.T. Spectroscopic Comparison of Effects of Electron Radiation on Mechanical Properties of Two Polyimides; NASA Report; NASA: Washington, DC, USA, 1987.
- Li, R.; Li, C.; He, S.; Di, M.; Yang, D. Radiation effect of keV protons on optical properties of aluminized Kapton film. Rad. Phys. Chem. 2007, 76, 1200–1204. [Google Scholar] [CrossRef]
- Choudhary, S.; Mungse, H.P.; Khatri, O.P. Dispersion of alkylated graphene in organic solvents and its potential for fabrication applications. J. Mater. Chem. 2012, 22, 21023–21039. [Google Scholar] [CrossRef]
- Lv, M.; Zheng, F.; Wang, Q.; Wang, T.; Liang, Y. Effect of proton irradiation on the friction and wear properties of polyimide. Wear 2014, 316, 30–36. [Google Scholar] [CrossRef]
- Sun, C.; Wu, Y.; Xiao, J.; Yu, S.; Yi, Z.; Shen, Z.; Wang, L.; Wang, Y. Proton flux effects and prediction on the free radicals behaviors of polyimide in vacuum using EPR measurements in ambient. Nucl. Instr. Meth. Phys. Res. B 2017, 397, 39–44. [Google Scholar] [CrossRef]
- Edwards, D.L.; de Groh, K.K.; Nehls, M.; Miller, S.K.; Banks, B.; Stephens, C.; Artiaga, R.; Benson, R.; Balascuta, S.; Zaleski, J.M.; et al. Spectroscopic investigations on the effect of proton bombardement of polyimide. Mater. Res. Soc. Symp. Proc. 2005, 851, NN 9.6.1. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, C.; Xiao, J.; Li, R.; Yang, D.; He, S. A study on the free-radical evolution and its correlation with the optical degradation of 170 keV proton-irradiated polyimide. Polym. Degr. Stab. 2010, 95, 1219–1225. [Google Scholar] [CrossRef]
- Sun, C.; Wu, Y.; Xiao, J.; Li, R.; Yang, D.; He, S. Pyrolytic carbon free-radical evolution and irradiation damage of polyimide under low energy proton irradiation. J. Appl. Phys. 2011, 110, 124909. [Google Scholar] [CrossRef]
- Sun, C.; Wu, Y.; Yue, L.; Sh, Y.; Xiao, J. Investigation on the recombination kinetics of the pyrolytic free-radicals in the irradiated polyimide. Nucl. Instr. Meth. Phys. Res. B 2012, 271, 61–64. [Google Scholar] [CrossRef]
- Hill, D.J.T.; Rasoull, F.A.; Forsythe, J.S.; O’Donnell, J.H.; Pomery, P.J.; George, G.A.; Young, P.R.; Connell, J.W. Effect of simulated low Earth orbit radiation on polyimides (UV degradation study). J. Appl. Polym. Sci. 1995, 58, 1847–1856. [Google Scholar] [CrossRef]
- Peng, G.R.; Hao, W.; Yang, D.; He, S. Degradation of polyimide film under vacuum ultraviolet irradiation. J. Appl. Polym. Sci. 2004, 94, 1370–1374. [Google Scholar] [CrossRef]
- George, M.A.; Ramakrishna, B.L.; Glaunsinger, W.S. Electron paramagnetic resonance investigation of free radicals in polyimide films. J. Phys. Chem. 1990, 94, 5159–5164. [Google Scholar] [CrossRef]
- Rasmussen, K.; Grampp, G.; van Eesbeek, M.; Rohr, T. Thermal and UV degradation of polymer films studied in situ with ESR spectroscopy. ACS Appl. Math. Int. 2012, 2, 1879–1883. [Google Scholar] [CrossRef]
- Wu, J.; Miyahara, A.; Khan, A.R.; Iwata, M.; Toyoda, K.; Cho, M.; Zheng, X.Q. Effects of ultraviolet irradiation and atomic oxygen erosion on total electron emission yield of polyimide. IEEE Trans. Plasma Sci. 2014, 42, 191–198. [Google Scholar] [CrossRef]
- Bungay, C.; Tiwald, T.E.; Devries, M.J.; Dworak, B.J.; Woollam, J.A. Characterization of UV irradiated space application polymers by spectroscopic ellipsometry. Polym. Eng. Sci. 2000, 40, 300–309. [Google Scholar] [CrossRef]
- Alegaonkar, P.S.; Bhoraskar, V.N.; Balaya, P.; Goyal, P.S. Dielectric properties of 1 MeV electron-irradiated polyimide. Appl. Phys. Lett. 2002, 80, 640. [Google Scholar] [CrossRef]
- Adamec, V. Temporary changes in electrical properties of polymer dielectrics due to ionizing radiation. J. Polym. Sci. 1968, 6, 1241–1253. [Google Scholar] [CrossRef]
- Seidl, T.; Plotnikov, A.; Mustafin, E.; Lopez, R.; Severin, D.; Floch, E.; Trautmann, C.; Golubev, A.; Smolyakov, A.; Tommastini, D.; et al. Influence of swift ion beams and protons on the dielectric strength of polyimide. Polym. Degr. Stab. 2012, 97, 2396–2402. [Google Scholar] [CrossRef]
- Shah, N.; Singh, N.L.; Desai, C.F.; Singh, K.P. Microhardness and radiation damage studies of proton irradiated Kapton films. Rad. Meas. 2003, 36, 699–702. [Google Scholar] [CrossRef]
- Woollam, J.A.; Bungay, C.; Hilfiker, J.; Tiwald, T. VUV and IR spectroellipsometric studies of polymer surfaces. Nucl. Inst. Meth. Phys. Res. B 2003, 208, 35–39. [Google Scholar] [CrossRef]
- Bolt, R.O.; Garrol, J.G. Radiation Effects on Organic Materials; Academic: New York, NY, USA, 1963. [Google Scholar]
- Mathakari, N.L.; Bhoraskar, V.N.; Dhole, S.D. MeV energy electron beam induced damage in isotactic polypropylene. Nucl. Instrum. Methods Phys. Res. B 2008, 266, 3075. [Google Scholar] [CrossRef]
- Bovey, F.A. The Effect of Ionizing Radiation on Natural and Synthetic High Polymers; Interscience: New York, NY, USA, 1958. [Google Scholar]
- Rahnamoun, A.; Engelhart, D.P.; Humagain, S.; Plis, E.; Kennedy, W.J.; Koerner, H.; Cooper, R.; Greenbaum, S.G.; Hoffmann, R.; van Duin, A.C.T. Chemical dynamics characteristics of Kapton polyimide damaged by electron beam irradiation. J. Chem. Phys. 2019. Submitted. [Google Scholar] [CrossRef]
- Mathakari, N.L.; Bhoraskar, V.N.; Dhole, S.D. 6MeV pulsed electron beam induced surface and structural changes in polyimide. Mater. Sci. Eng. B 2010, 168, 122–126. [Google Scholar] [CrossRef]
- Shen, Z.; Mu, Y.; Ding, Y.; Liu, Y.; Zhao, C. Study on the mechanical property of polyimide film in space radiation environments. In Selected Papers of the Photoelectronic Technology Committee Conferences Held November 2015; SPIE Press: Bellingham, DC, USA, 2016. [Google Scholar] [CrossRef]
- Tsai, F.-Y.; Kuo, Y.-H.; Harding, D.R. Properties and structure of vapor-deposited polyimide upon electron-beam irradiation. J. Appl. Phys. 2006, 99, 64910. [Google Scholar] [CrossRef]
- Zicai, S.; Yuming, L.; Weiquan, F.; Chunqing, Z.; Yigang, D. Degradation of mechanical properties of spacecraft polyimide film exposed to radiation environments. Astrophys. Space Sci. Proc. 2013, 32, 469–482. [Google Scholar] [CrossRef]
- Spindel, A.; Reed, R.P.; Tupper, M.; Darr, J.; Pollock, D. Low-temperature electron irradiation of insulating films and adhesives. In Advances in Cryogenic Engineering Materials; Reed, R.P., Fickett, F.R., Summers, L.T., Stieg, M., Eds.; An International Cryogenic Materials Conference Publication, vol 40; Springer: Boston, MA, USA, 2018. [Google Scholar]
- Sasuga, T.; Hayakawa, N.; Yoshida, K.; Hagiwara, M. Degradation in tensile properties of aromatic polymers by electron beam irradiation. Polymer 1985, 86, 1039–1045. [Google Scholar] [CrossRef]
- Kang, P.-H.; Jeon, Y.-K.; Jeun, J.-P.; Shin, J.-W.; Nho, Y.-C. Effect of electron beam irradiation on polyimide film. J. Ind. Eng. Chem. 2008, 14, 672–675. [Google Scholar] [CrossRef]
- Pei, X.; Wang, Q. Effect of Electron Radiation on the Tribological Properties of Polyimide. Tribol. Trans. 2007, 50, 268–272. [Google Scholar] [CrossRef]
- Hill, D.J.T.; Hopewell, J.L. Effects of 3 MeV proton irradiation on the mechanical properties of polyamide films. Radiat. Phys. Chem. 1996, 48, 533–537. [Google Scholar] [CrossRef]
- Kim, D.-W.; Kim, D.-I.; Huh, Y.-H.; Yang, T.-K.; Kim, Y.-S.; Ha, M.-G.; Won, M.-S.; Kim, K.-R.; Lee, J.-H. Influence of MeV protons on mechanical properties of ITO/aluminum-coated Kapton designed foe space missions. Nucl. Instr. Meth. Phys. Res. B 2008, 266, 3263–3274. [Google Scholar] [CrossRef]
- Dever, J.A.; Messer, R.; Powers, C.; Townsend, J.; Wooldrifge, E. Effects of vacuum ultraviolet radiation on thin polyimide films. High Perform. Polym. 2001, 13, S391–S399. [Google Scholar] [CrossRef]
- Russell, D.A.; Fogdall, L.B.; Bohnhoff-Hlavacek, G. Simulated Space Environmental Testing on Thin Films; NASA/CR-2000-210101; NASA: Washington, DC, USA, 2000.
- Li, R.; Li, C.; He, S.; Di, M.; Yang, D. Damage effect of the keV proton irradiation on aluminized Kapton film. Rad. Phys. Chem. 2008, 77, 482–489. [Google Scholar] [CrossRef]
- Ennis, C.P.; Kaiser, R.I. Mechanistical studies on the electron-induced degradation of polymethylmethacrylate and Kapton. Phys. Chem. Chem. Phys. 2010, 12, 14902–14915. [Google Scholar] [CrossRef]
- Mishra, R.; Tripathy, S.P.; Sinha, D.; Dwivedi, K.K.; Shosh, S.; Khathing, D.T.; Muller, M.; Fink, D.; Chung, W.H. Optical and electrical properties of some electron and proton irradiated polymers. Nucl. Ins. Meth. Phys. Res. B 2000, 168, 59–64. [Google Scholar] [CrossRef]
- Engelhart, D.P.; Plis, E.; Cooper, R.; Humagain, S.; Koch, A.; Brunetti, M.; Greenbaum, S.; Hoffmann, R. Effect of Electron Bombardment on Polyimide Film. Key Eng. Mater. 2018, 759, 48–53. [Google Scholar] [CrossRef]
- Castle, J.G. Measurements of Material Properties for Solar Cells; NASA Report NASA-CR-150797; NASA: Washington, DC, USA, 1978. Available online: https://ntrs.nasa.gov/search.jsp?R=19780023588 (accessed on 9 January 2019).
- Rai, V.N. Optical properties of neutron and proton irradiated Kapton foil. Appl. Opt. 1989, 28, 2450–2451. [Google Scholar] [CrossRef]
- Ishida, H.; Wellinghoff, S.T.; Baer, E.; Koenig, J.L. Spectroscopic Studies of Poly[N,N′-bis(phenoxyphenyl)pyromellitimide]. 1. Structures of the Polyimide and Three Model Compounds. Macromolecules 1980, 13, 826–834. [Google Scholar] [CrossRef]
- LaFemina, J.P.; Arjavalingam, G.; Hougham, G. Electronic structure and ultraviolet absorption spectrum of polyimide. J. Chem. Phys. 1989, 90, 5154–5160. [Google Scholar] [CrossRef]
- Komiyama, Y.; Miyake, H.; Tanaka, Y.; Takada, T. Observation of Surface Discharge Phenomena on Dielectric Films Under Low Pressure Using Pockels Effect in Protection of Materials and Structures from the Space Environment; Kleiman, M.T.J., Kimoto, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; p. 456. [Google Scholar]
- Sun, Y.M.; Zhu, Z.Y.; Jin, Y.F.; Liu, C.L.; Wang, Z.G.; Zhang, Q.X. The effects of high electronic energy loss on the chemical modification of polyimide. Nucl. Instrum. Meth. B 2002, 193, 214–220. [Google Scholar] [CrossRef]
- Shi, J.; Gong, C.; Tian, X.; Yang, S.; Chu, P.K. Optical properties and chemical structures of Kapton-H film after proton irradiation by immersion in a hydrogen plasma. Appl. Surf. Sci. 2012, 258, 3829–3834. [Google Scholar] [CrossRef]
- Alegaonkar, P.S.; Bhoraskar, V.N. Characterization of polyimide irradiated in silver solution with a pulsed electron beam. Nucl. Insr. Meth. Phys. Res. B 2004, 225, 267–274. [Google Scholar] [CrossRef]
- Mishra, R.; Triphany, S.P.; Dwivedi, K.K.; Khathing, D.T.; Ghosh, S.; Muller, M.; Fink, D. Modification in etching characteristics and surface topography of some electron irradiated polymers. Radiat. Meas. 2001, 34, 95–98. [Google Scholar] [CrossRef]
- Coulter, D.R.; Gupta, A.; Smith, M.V. The Effects of Energetic Proton Bombardment on Polymeric Materials: Experimental Studies and Degradation Models; NASA Report 19860018724; NASA: Washington, DC, USA, 1986. Available online: https://ntrs.nasa.gov/search.jsp?R=19860018724 (accessed on 8 September 2018).
- Himmelbauer, M.; Arenholz, E.; Bäuerle, D.; Schilcher, K. UV-laser-induced surface topology changes in polyimide. Appl. Phys. A 1996, 63, 337–339. [Google Scholar] [CrossRef]
- Mishra, R.; Tripathy, S.P.; Dwivedi, K.K.; Khathing, D.T.; Ghosh, S.; Muller, M.; Fink, D. Spectroscopic and thermal studies of electron irradiated polyimide. Radiat. Meas. 2003, 36, 621–624. [Google Scholar] [CrossRef]
- Mishra, R.; Tripathy, S.P.; Dwivedi, K.K.; Khathing, D.T.; Ghosh, S.; Fink, D. Dose-dependent modification in makrofol-N and polyimide by proton irradiation. Rad. Meas. 2003, 36, 719–722. [Google Scholar] [CrossRef]
- Alegaonkar, P.S.; Bhoraskar, V.N. Effect of MeV electron irradiation on the free volume of polyimide. Radiat. Eff. Defects Solids 2004, 159, 511–516. [Google Scholar] [CrossRef]
- Hirade, T.; Oka, T.; Morishita, N.; Idesaki, A.; Shimada, A. Positron annihilation lifetime of irradiated polyimide. Mater. Sci. Forum. 2013, 733, 151–154. [Google Scholar] [CrossRef]
- Mahapatra, S.K.; Dhole, D.D.; Bhoraskar, V.N. Growth and decay of surface voltage on silver diffused polyimide exposed to 3–15 keV electrons. J. Phys. D Appl. Phys. 2007, 40, 1097–1102. [Google Scholar] [CrossRef]
- Mahapatra, S.K.; Dhole, S.D.; Bhoraskar, V.N. Dependence of charge buildup in the polyimide on the incident electron energy. J. Appl. Phys. 2006, 100, 34913. [Google Scholar] [CrossRef]
- Clough, R.L. High-energy radiation and polymers: A review of commercial processes and emerging applications. Nucl. Inst. Meth. Phys. Res. B 2001, 185, 8–33. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mahajan, J.; Bhattacharyyalia, P.K. Electron beam (Eb) crosslinking of PVC insulation in presence of sensitiser additives. Rad. Phys. Chem. 1995, 45, 695–701. [Google Scholar] [CrossRef]
- Zimek, Z.; Przybytniak, G.; Nowicki, A. Optimization of electron beam crosslinking of wire and cable insulation. Radiat. Phys. Chem. 2012, 81, 1398–1403. [Google Scholar] [CrossRef]
- Dadbin, S.; Frounchi, M.; Saeid, M.H.; Gangi, F. Molecular structure and physical properties of E-beam crosslinked low-density polyethylene for wire and cable insulation applications. J. Appl. Polym. Sci. 2002, 86, 1959–1969. [Google Scholar] [CrossRef]
- Ramachandran, P.; Naskar, K.; Nando, G.B. Exploring the effect of radiation crosslinking on the physico-mechanical, dynamic mechanical and dielectric properties of EOC–PDMS blends for cable insulation applications. Polym. Adv. Tech. 2017, 28, 80–93. [Google Scholar] [CrossRef]
- Min, J.; Tao, Z.; Song, X.; Zhang, J.; Wang, Z.; Ji, Y.; Zhai, G.; Ye, X. Study on electron beam cross-linked ethylene-tetrafluoroethylene copolymer-insulated cables for aerospace applications: Mechanical performance, crystallization kinetics, and fluoride precipitation. Polym. Adv. Tech. 2018, 29, 1497–1506. [Google Scholar] [CrossRef]
- Cleland, M.R.; Parks, L.A.; Cheng, S. Applications for radiation processing of materials. Nucl. Inst. Meth. Phys. Res. B 2003, 208, 66–73. [Google Scholar] [CrossRef]
- Mishra, J.K.; Chang, Y.W.; Lee, B.C.; Ryu, S.H. Mechanical properties and heat shrinkability of electron beam crosslinked polyethylene–octene copolymer. Rad. Phys. Chem. 2008, 77, 675–679. [Google Scholar] [CrossRef]
- Yang, S.L.; Wu, Z.H.; Yang, W.; Yang, M.B. Thermal and mechanical properties of chemical crosslinked polylactide (PLA). Polym. Test. 2008, 27, 957–963. [Google Scholar] [CrossRef]
- Berg, G.J.; McBride, M.K.; Wang, C.; Bowman, C.N. New directions in the chemistry of shape memory polymers. Polymer 2014, 55, 5849–5872. [Google Scholar] [CrossRef]
- Chernous, D.A.; Shil’ko, S.V.; Pleskachevskii, Y.M. Description of the Shape Memory Effect of Radiation-Modified Polymers under Thermomechanical Action. J. Eng. Phys. Thermophys. 2004, 77, 6–10. [Google Scholar] [CrossRef]
- Hearon, K.; Nash, L.D.; Volk, B.L.; Ware, T.; Lewicki, J.P.; Voit, W.E.; Wilson, T.S.; Maitland, D.J. Electron beam crosslinked polyurethane shape memory polymers with tunable mechanical properties. Macromol. Chem. Phys. 2013, 214, 1258–1272. [Google Scholar] [CrossRef]
- Xie, F.; Liu, L.; Gong, X.; Huang, L.; Leng, J.; Liu, Y. Effects of accelerated aging on thermal, mechanical and shape memory properties of cyanate-based shape memory polymer: I vacuum ultraviolet radiation. Polym. Degr. Stab. 2017, 138, 91–97. [Google Scholar] [CrossRef]
- Hou, L.; Wu, Y.; Guo, B.; Shan, D.; Zong, Y. Degeneration and damage mechanism of epoxy-based shape memory polymer under 1 MeV electron irradiation. Mater. Lett. 2018, 222, 37–40. [Google Scholar] [CrossRef]
- Gao, H.; Lan, X.; Liu, L.; Xiao, X.; Liu, Y.; Leng, J. Study on performances of colorless and transparent shape memory polyimide film in space thermal cycling, atomic oxygen and ultraviolet irradiation environments. Smart. Mater. Struct. 2017, 26, 095001. [Google Scholar] [CrossRef]
- Oshima, A.; Shiraki, F.; Fujita, H.; Washio, M. Surface modification of polymeric materials using ultra low energy electron beam irradiation. Rad. Phys. Chem. 2011, 80, 196–200. [Google Scholar] [CrossRef]
- Srinivasan, R.; Braren, B. Ultraviolet laser ablation of organic polymers. Chem. Rev. 1989, 89, 1303–1316. [Google Scholar] [CrossRef]
- Hiraoka, H.; Lazare, S. Surface modifications of Kapton and cured polyimide films by ArF excimer laser: Applications to imagewise wetting and metallization. Appl. Surf. Sci. 1990, 46, 264–271. [Google Scholar] [CrossRef]
- Adhi, K.P.; Owings, R.L.; Railkar, T.A.; Brown, W.D.; Malshe, A.P. Chemical modifications in femtosecond ultraviolet (248 nm) excimer laser radiation-processed polyimide. Appl. Surf. Sci. 2004, 225, 324–331. [Google Scholar] [CrossRef]
- Du, Q.; Liu, J.; Guo, L.; Lv, M.; Zeng, X. Tailoring the surface wettability of polyimide by UV laser direct texturing in different gas atmospheres. Mater. Des. 2016, 104, 134–140. [Google Scholar] [CrossRef]
- Du, Q.; Chen, T.; Liu, J.; Zeng, X. Surface microstructure and chemistry of polyimide by single pulse ablation of picosecond laser. Appl. Surf. Sci. 2018, 434, 588–595. [Google Scholar] [CrossRef]
- Lorenz, P.; Lorenz, P.; Bayer, L.; Bayer, L.; Ehrhardt, M.; Ehrhardt, M.; Zimmer, K.; Zimmer, K.; Engisch, L. Nanosecond laser-induced ablation and laser-induced shockwave structuring of polymer foils down to sub-μm patterns. Proc. SPIE 2015, 9351, 935119. [Google Scholar] [CrossRef]
- Reddy, M.R.; Srinivasamurthy, N.; Agrawal, B.L. Atomic oxygen protective coatings for Kapton film: A review. Surf. Coat. Tech. 1993, 58, 1. [Google Scholar] [CrossRef]
- Schuler, P.; Mojazza, H.B.; Haghighat, R. Atomic oxygen resistant films for multi-layer insulation. High Perform. Polym. 2000, 12, 113. [Google Scholar] [CrossRef]
- Devapal, D.; Packirisamy, S.; Korulla, R.M.; Ninan, K.N. Atomic oxygen resistant coating from poly (tetramethyldisilylene-co-styrene). J. Appl. Polym. Sci. 2004, 94, 2368. [Google Scholar] [CrossRef]
- Duo, S.; Li, M.; Zhu, M.; Zhou, Y. Polydimethylsiloxane/silica hybrid coatings protecting Kapton from atomic oxygen attack. Mater. Chem. Phys. 2008, 112, 1093. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Liu, G.; He, S.; Yang, D. Investigation on sol–gel boehmite-AlOOH films on Kapton and their erosion resistance to atomic oxygen. Thin Solid Film. 2008, 516, 5020. [Google Scholar] [CrossRef]
- Hu, L.; Li, M.; Xu, C.; Luo, Y. Perhydropolysilazane derived silica coating protecting Kapton from atomic oxygen attack. Thin Solid Film. 2011, 520, 1063. [Google Scholar] [CrossRef]
- Qi, H.; Qian, Y.; Xu, J.; Li, M. Studies on atomic oxygen erosion resistance of deposited Mg-alloy coating on Kapton. Corros. Sci. 2017, 124, 56. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Qian, Y.; Qi, H.; Li, J.; Sun, J. Mechanically Robust Atomic Oxygen-Resistant Coatings Capable of Autonomously Healing Damage in Low Earth Orbit Space Environment. Adv. Mater. 2018, 30, 1803854. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Qian, Y.; Xu, J.; Zuo, J.; Li, M. An AZ31 magnesium alloy coating for protecting polyimide from Erosion-Corrosion by Atomic Oxygen. Corros. Sci. 2018, 138, 170. [Google Scholar] [CrossRef]
- Sibin, K.P.; Esther, A.C.; Shashikala, H.D.; Dey, A.; Sridhara, N.; Sharma, A.K.; Barshilia, H.C. Environmental stability of transparent and conducting ITO thin films coated on flexible FEP and Kapton® substrates for spacecraft applications. Sol. Energy Mater. Sol. Cell. 2018, 176, 134. [Google Scholar] [CrossRef]
- Connell, J.W.; Smith, J.G., Jr.; Hergenrother, P.M. The effect of oxygen plasma on some experimental polymers containing silicon and phosphorus. J. Fire Sci. 1993, 11, 137. [Google Scholar] [CrossRef]
- Connell, J.W. The effect of low earth orbit atomic oxygen exposure on phenylphosphine oxide-containing polymers. High Perform. Polym. 2000, 12, 43. [Google Scholar] [CrossRef]
- Devapal, D.; Packirisamy, S.; Nair, C.R.; Ninan, K.N. Phosphazene-based polymers as atomic oxygen resistant materials. J. Mater. Sci. 2006, 41, 5764. [Google Scholar] [CrossRef]
- Liu, B.; Ji, M.; Liu, J.; Fan, L.; Yang, S. Phenylphosphine oxide containing polyimide matrix resins for atomic oxygen-resistant fiber-reinforced composites. High Perform. Polym. 2013, 25, 907. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, K.; Zhan, M.S. Atomic oxygen resistant phosphorus-containing polyimides for LEO environment. J. Mater. Sci. 2012, 47, 4904. [Google Scholar] [CrossRef]
- Su, L.; Tao, L.; Wang, T.; Wang, Q. Phenylphosphine oxide-containing aromatic polyamide films with high atomic oxygen erosion resistance. Polym. Degrad. Stab. 2012, 97, 981. [Google Scholar] [CrossRef]
- Song, G.; Li, X.; Jiang, Q.; Mu, J.; Jiang, Z. A novel structural polyimide material with synergistic phosphorus and POSS for atomic oxygen resistance. RSC Adv. 2015, 5, 11980. [Google Scholar] [CrossRef]
- Wei, J.H.; Gang, Z.X.; Ming, L.Q.; Wei, Z.H.; Dong, D.G.; Hai, C.C. Atomic oxygen resistant phosphorus-containing copolyimides derived from bis [4-(3-aminophenoxy) phenyl] phenylphosphine oxide. Polym. Sci. Ser. B. 2014, 56, 788. [Google Scholar] [CrossRef]
- Chunbo, W.; Haifu, J.; Dongbo, T.; Wei, Q.; Chunhai, C.; Xiaogang, Z.; Hongwei, Z.; Daming, W. Atomic oxygen effects on polymers containing silicon or phosphorus: Mass loss, erosion yield, and surface morphology. High Perform. Polym. 2018. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, H.; Li, G.M.; Liu, F.F.; Dai, X.M.; Dong, Z.X.; Qiu, X.P. Synthesis and AO Resistant Properties of Novel Polyimide Fibers Containing Phenylphosphine Oxide Groups in Main Chain. Chin. J. Polym. Sci. 2019, 37, 59. [Google Scholar] [CrossRef]




| Assignment (cm−1) | Irradiation Absorption (cm−1) | Characterization |
|---|---|---|
| 568 | δ(phenyl) | Phenyl ring deformation |
| 607 | δ(phenyl) | Phenyl ring deformation |
| 636 | δ(C=O) | Carbonyl deformation |
| 705 | δ(C-N-C) | Imide deformation |
| 717 | C=O | Carbonyl bending |
| 725 | δ(C-N-C) | Out-of-phase bending of imide ring |
| 734 | C=O | Carbonyl bending |
| 752–960 | δ(phenyl) | Phenyl ring deformation |
| 1079 | C-O-C stretch | Stretching deformation |
| 1095 | v(C-N-C) | Imide stretch |
| 1117 | v(C-N-C) | Imide stretch |
| 1160 | C-C | C-C bending |
| 1168 | v(C-N-C) | Imide stretch |
| 1188 | δ(phenyl) | Phenyl ring deformation |
| 1230 | C-N | C-N stretch |
| 1243 | v(C-O-C) | Bridging C-O-C stretch |
| 1260 | v(C-O-C) | Bridging C-O-C stretch |
| 1290–1307 | δ(phenyl) | Phenyl ring deformation |
| 1350 | C-N | C-N stretch |
| 1376 | δ(C-N-C) | Imide stretch |
| 1390 | C-N | C-N stretch |
| 1456–1600 | v(phenyl) | Phenyl ring C-C stretch |
| 1679–1779 | v(C=O) | Carbonyl stretch |
| 1825 | Cyclic anhydrides, presented in not fully cured polymer (precurser) | |
| 3020–3155 | v(C-H) | Phenyl ring C-H stretch |
| 3488 | v(C-H) | Phenyl ring C-H stretch |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plis, E.A.; Engelhart, D.P.; Cooper, R.; Johnston, W.R.; Ferguson, D.; Hoffmann, R. Review of Radiation-Induced Effects in Polyimide. Appl. Sci. 2019, 9, 1999. https://0-doi-org.brum.beds.ac.uk/10.3390/app9101999
Plis EA, Engelhart DP, Cooper R, Johnston WR, Ferguson D, Hoffmann R. Review of Radiation-Induced Effects in Polyimide. Applied Sciences. 2019; 9(10):1999. https://0-doi-org.brum.beds.ac.uk/10.3390/app9101999
Chicago/Turabian StylePlis, Elena A., Daniel P. Engelhart, Russell Cooper, W. Robert Johnston, Dale Ferguson, and Ryan Hoffmann. 2019. "Review of Radiation-Induced Effects in Polyimide" Applied Sciences 9, no. 10: 1999. https://0-doi-org.brum.beds.ac.uk/10.3390/app9101999