Novel Beverages of Yerba-Mate and Soy: Bioactive Compounds and Functional Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Beverages
2.2. Chemical Composition
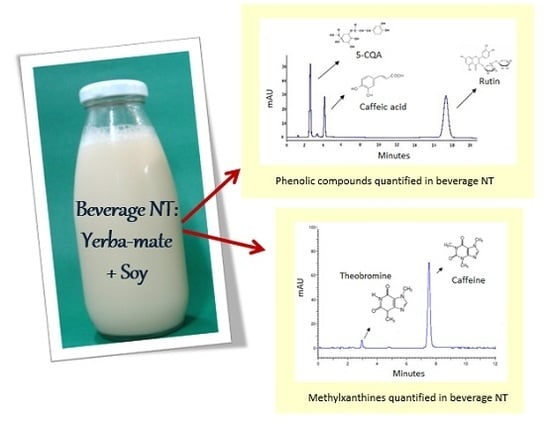
2.2.1. Phenolic Compounds
2.2.2. Methylxanthines
2.3. Antioxidant Activity
2.3.1. ABTS•+ Radical Method
2.3.2. DPPH• Radical Method
2.4. Physical Characterization
Color
2.5. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition and Physico-Chemical Characteristics of Yerba-Mate (Native and Planted) and Beverages
3.2. Polyphenol Content and Antioxidant Activity
3.3. Methylxanthines
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rotta, E.; Oliveira, Y.M.M. Cultivo da Erva-Mate; Sistemas de produção n.1.; Empresa Brasileira de Pesquisa Agropecuária - Embrapa: Colombo, Brazil, 2005. (In Portuguese) [Google Scholar]
- Filip, R.; Davicino, R.; Anesini, C. Antifungal activity of the aqueous extract of llex paraguariensis against Malassezia furfur. Phytother. Res. 2010, 24, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Suzan, A.J.; Cerutti, S.M.; Arçari, D.P.; Ribeiro, M.L.; Bastos, D.H.; Carvalho Pde, O. Consumption of mate tea (llex paraguariensis) decreases the oxidation of unsaturated fatty acids in mouse liver. Br. J. Nutr. 2009, 4, 527–532. [Google Scholar] [CrossRef]
- Gugliucci, A. Antioxidant effects of llex paraguariensis, induction of decreased oxidability of human LDL in vivo. Biochem. Biophys. Res. Commun. 1996, 224, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Bracesco, N.; Dell, M.; Rocha, A.; Behtash, S.; Menini, T.; Gugliucci, A.; Nunes, E. Antioxidant activity of a botanical extract preparation of llex paraguariensis, prevention of DNA double-strand breaks in Saccharomyces cerevisiae and human low-density lipoprotein oxidation. J. Altern. Complem. Med. 2003, 9, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.D.C.; Arçari, D.P.; Pedrazzoli, J., Jr.; Carvalho, P.O.; Cerutti, S.M.; Bastos, D.H.; Ribeiro, M.L. Protective effects of mate tea (llex paraguariensis) on H2O2-induced DNA damage and DNA repair in mice. Mutagenesis 2008, 23, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Puangpraphant, S.; Berhow, M.A.; de Mejia, E.G. Mate (llex paraguariensis St. Hilaire) saponins induce caspase-3-dependent apoptosis in human colon cancer cells in vitro. Food Chem. 2011, 125, 1171–1178. [Google Scholar] [CrossRef]
- Boaventura, B.C.B.; Di Pietro, P.F.; Stefanuto, A.; Klein, G.A.; Morais, E.C.M.; Andrade, F.; Wazlawik, E.; Silva, E.L. Association of mate tea (llex paraguariensis) intake and dietary intervention and effects on oxidative stress biomarkers of dyslipidemic subjects. Nutrition 2012, 28, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.A. Cultura Da Soja; Cinco Continentes: Porto Alegre, Brazil, 1996; p. 233. (In Portuguese) [Google Scholar]
- EmpresaBrasileira de Pesquisa Agropecuária (Embrapa). Soja transgênica. 2006. Available online: http://www.cnpso.embrapa.br (accessed on 2 January 2017).
- Frizon, C.N.T.; Perussello, C.A.; Sturion, J.S.; Fracasso, A.F.; Hoffmann-Ribani, R. Stability of beverages of yerba-mate (llex paraguariensis) with soy. Nutr. Food Sci. 2015, 45, 467–478. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of the AOAC International, 18th ed.; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Watt, B.; Merrill, A.L. Composition of Foods: Raw, Processed, Prepared; Consumer and Food Economics Research; Research Service, U.S. Department of Agriculture: Washington, DC, USA, 1999.
- Dutra, F.L.G.; Hoffmann-Ribani, R.; Ribani, M. Determinação de compostos fenólicos por cromatografia líquida de alta eficiência isocrática durante estacionamento da erva-mate. Quím. Nova 2010, 33, 119–123. (In Portuguese) [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Hunterlab. CIE L*a*b* Color Scale. 2008. Available online: http://hunterlab.com (accessed on 5 January 2015).
- Berté, K.A.; Beux, M.R.; Spada, P.K.; Salvador, M.; Hoffman-Ribani, R. Chemical composition and antioxidant activity of yerba-mate (llex paraguariensis A. St.-Hil., Aquifoliaceae) extract as obtained by spray drying. J. Agric. Food Chem. 2011, 59, 5523–5527. [Google Scholar] [CrossRef]
- Esmelindro, M.C.; Toniazzo, G.; Waczuk, A.; Dariva, C.; Oliveira, D. Caracterização físico-química da erva-mate: Influência das etapas do processamento industrial. Ciência e Tecnologia de Alimentos 2002, 22, 199–204. (In Portuguese) [Google Scholar] [CrossRef]
- Valduga, E.; Freitas, R.J.S.; Reismann, C.B.; Nakashima, T. Caracterização química da folha de llex paraguariensis St. Hil. (erva-mate) e de outras espécies utilizadas na adulteração do mate. Boletim do Centro de Processamento de Alimentos 1997, 15, 25–36. (In Portuguese) [Google Scholar]
- Esmelindro, A.A.; Girardi, J.S.; Mossi, A.; Jacques, R.A.; Dariva, C. Influence of agronomic variables on the composition of mate tea leaves (llex paraguariensis) extracts obtained from CO2 extraction at 30 °C and 175 bar. J. Agric. Food Chem. 2004, 52, 1990–1995. [Google Scholar] [CrossRef] [PubMed]
- Gnoatto, S.C.B.; Bassani, V.L.; Coelho, G.C.; Schenkel, E.P. Influência do método de extração nos teores de metilxantinas em erva-mate (llex paraguariensis A. St.-Hil. Aquifoliaceae). Quím. Nova 2007, 30, 304–307. (In Portuguese) [Google Scholar] [CrossRef]
- Blum-Silva, C.H.; Luz, A.B.G.; Nascimento, M.V.P.S.; Facchin, B.M.C.; Baratto, B.; Fröde, T.S.; Sandijo, P.L.; Dalmarco, E.M.; Reginatto, F.H. Qualitative and quantitative analysis data of the major constituents of llex paraguariensis leaves by UPLC-PDA and QTOF-MS. Data Brief 2016, 8, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Butiuk, A.P.; Martos, M.A.; Adachi, O.; Hours, R.A. Study of the chlorogenic acid content in yerba mate (llex paraguariensis St. Hil.): Effect of plant fraction, processing step and harvesting season. J. Appl. Res. Med. Aromat. Plants 2016, 3, 27–33. [Google Scholar] [CrossRef]
- Potter, R.M.; Dougherty, M.P.; Halteman, W.A.; Camire, M.E. Characteristics of wild blueberry-soy beverages. Food Sci. Technol. LEB 2007, 40, 807–814. [Google Scholar] [CrossRef]
- Mello, A.C.B.; Freitas, R.J.S.; Waszczynskyj, N.; Koehler, H.S.; Wille, G.F.C.; Berté, K.A.S. Bebida gaseificada de erva-mate verde. Boletim do Centro de Processamento de Alimentos 2009, 27, 19–26. (In Portuguese) [Google Scholar] [CrossRef]
- Contreras, P.D. Desenvolvimento de Bebida à Base de Subprodutos da Indústria da Erva-Mate (llex paraguariensis St. Hil.) e Verificação de Sua Atividade Antioxidante. Universidade Federal do Paraná, Curitiba-PR. 2007. Available online: http://dspace.c3sl.ufpr.br/dspace/handle/1884/13514 (accessed on 26 June 2015). (In Portuguese).
- Dartora, N.; de Souza, L.M.; Santana-Filho, A.P.; Iacomini, M.; Valduga, A.T.; Gorin, P.A.J.; Sassaki, G.L. UPLC-PDA–MS evaluation of bioactive compounds from leaves of llex paraguariensis with different growth conditions, treatments and ageing. Food Chem. 2011, 129, 453–1461. [Google Scholar] [CrossRef]
- Blum-Silva, C.H.; Chaves, V.C.; Schenkel, E.P.; Coelho, G.C.; Reginatto, F.H. The influence of leaf age on methylxanthines, total phenolic content, and free radical scavenging capacity of llex paraguariensis aqueous extracts. Rev. Bras. Farmacogn. 2015, 25. [Google Scholar] [CrossRef]
- Strassmann, B.B.; Vieira, A.R.; Pedrotti, E.L.; Morais, H.N.F.; Dias, P.F.; Maraschin, M.N.N. Quantitation of methylxanthinic alkaloids and phenolic compounds in mate (llex paraguariensis) and their effects on blood vessel formation in chick embryos. J. Agric. Food Chem. 2008, 58, 8348–8353. [Google Scholar] [CrossRef] [PubMed]
- Bieza, K.; Lois, R. An Arabidopsis mutant tolerant to lethal ultraviolet-b levels shows constitutively elevated accumulation of flavonoids and other phenolics. Plant Physiol. 2001, 126, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Agati, G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 2004, 163, 547–561. [Google Scholar] [CrossRef]
- Marques, V.; Farah, A. Chlorogenic acids and related compounds in medicinal plants and infusions. Food Chem. 2009, 113, 1370–1376. [Google Scholar] [CrossRef]
- Bastos, D.H.M.; Fornari, A.C.; Queiroz, Y.S.; Soares, R.A.M.; Torres, E.A.F.S. The chlorogenic acid and caffeine content of yerba maté (llex paraguariensis) beverages. Acta Farm. Bonaer. 2005, 24, 91–95. [Google Scholar]
- Duarte, G.S.; Farah, A. Effect of simultaneous consumption of milk and coffee on chlorogenic acids bioavailability in humans. J. Agric. Food Chem. 2011, 59, 7925–7931. [Google Scholar] [CrossRef] [PubMed]
- Bastos, D.H.M.; Fornari, A.C.; Queiroz, Y.S.; Torres, E.A.F.S. Bioactive compounds content of chimarrão infusions related to the moisture of yerba maté (llex Paraguariensis) leaves. Braz. Arch. Biol. Technol. 2006, 49, 399–404. [Google Scholar] [CrossRef]
- Nevena, G.L.; Branislava, R.; Emilia, S.; Dusica, R.; Ivan, N.; Nebojsa, K.; Biljana, B. Determination of 5-caffeoylquinic acid (5-CQA) as one of the major classes of chlorogenic acid in commercial tea and coffee samples. Vojnosanit. Pregl. 2015, 72, 1018–1023. [Google Scholar] [PubMed]
- Mezadri, T.; Villaño, D.; Fernandez-Páchon, M.S.; García-Parrilla, M.C.; Troncoso, A.M. Antioxidant compounds and antioxidant activity in acerola (Malpighiaemarginata DC.) fruits and derivatives. J. Food Compos. Anal. 2008, 21, 282–290. [Google Scholar] [CrossRef]
- Campos, A.M.; Lissi, E.A. Kinetics of the reaction between 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) derived radical cations and phenols. Int. J. Chem. Kinet. 1997, 29, 219–224. [Google Scholar] [CrossRef]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicatíon de diversos métodos químicos para determiner actividad antioxidante en pulpa de frutos. Ciência e Tecnologia de Alimentos 2005, 25, 726–732. (In Spanish) [Google Scholar] [CrossRef]
- Bixby, M.; Spieler, L.; Menini, T.; Gugliucci, A. Ilex paraguariensis extracts are potent inhibitors of nitrosative stress: A comparative study with green tea and wines using a protein nitration model and mammalian cell cytotoxicity. Life Sci. 2005, 77, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Anesini, C.; Turner, S.; Cogoi, L.; Filip, R. Study of the participation of caffeine and polyphenols on the overall antioxidant activity of mate (llex paraguariensis). Food Sci. Technol. LEB 2012, 45, 299–304. [Google Scholar] [CrossRef]
- Xiang, T.; Xiong, Q.B.; Ketut, A.I.; Tezuka, Y.; Nagaoka, T.; Wu, L.J.; Kadota, S. Studies on the hepatocyte protective activity and the structure-activity relationships of quinic acid and caffeic acid derivatives from the flower buds of Lonicerabournei. Planta Med. 2001, 67, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Namalakkannan, N.; Prince, P.S. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin. Pharmacol. 2006, 98, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Coelho, G.C.; Rachwal, M.F.G.; Dedecek, R.A.; Curcio, G.R.; Nietschem, K.; Schenkel, E.P. Effect of light intensity on methylxanthine contents of llex paraguariensis A. St. Hil. Biochem. Syst. Ecol. 2007, 35, 75–80. [Google Scholar] [CrossRef]
- Heck, C.I.; Mejia, E.G. Yerba-mate tea (llex paraguariensis): A comprehensive review on chemistry, health implications, and technological considerations. J. Food Sci. 2007, 72, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Meinhart, A.D.; Bizzotto, C.S.; Ballus, C.A.; Rybka, A.C.P.; Sobrinho, M.R.; Cerro-Quintana, R.S.; Teixeira-Filho, J.; Godoy, H.T. Methylxanthines and phenolics content extracted during the consumption of mate (llex paraguariensis St. Hil) beverages. J. Agric. Food Chem. 2010, 58, 2188–2193. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.N. Maté: A risk factor for oral and or pharyngeal cancer. Oral Oncol. 2002, 38, 646–649. [Google Scholar] [CrossRef]

| Parameter | Yerba-Mate Leaves | Beverage | ||
|---|---|---|---|---|
| PL | NT | PL | NT | |
| Proteins (g.100 g−1) | 9.52 ± 0.42 a | 10.06 ± 0.12 a | 0.33 ± 0.31 c | 0.52 ± 0.39 b |
| Fats (g.100 g−1) | 6.30 ± 0.06 b | 8.13 ± 0.10 a | 0.95 ± 0.19 d | 1.33 ± 0.03 c |
| Moisture (g.100 g−1) | 5.77 ± 0.07 c | 5.70 ± 0.09 c | 90.60 ± 0.21 a | 88.19 ± 0.10 b |
| Ashes (g.100 g−1) | 7.04 ± 0.31 a | 7.01 ± 0.07 a | 0.21 ± 0.01 b | 0.22 ± 0.01 b |
| pH | - | - | 4.36 ± 0.02 b | 4.46 ± 0.01 a |
| Edible fibers (g.100 g−1) | - | - | 0.41 ± 0.03 b | 0.54 ± 0.04 a |
| Total soluble solids (°Brix) | - | - | 8.11 ± 0.02 b | 10.16 ± 0.10 a |
| Carbohydrates (g.100 mL) | - | - | 7.50 ± 0.08 b | 9.20 ± 0.10 a |
| Energy (kcal/100 mL) | - | - | 36.20 ± 0.21 b | 48.42 ± 0.23 a |
| Luminosity (L*) | 36.95 ± 0.59 d | 42.14 ± 0.72 c | 60.19 ± 0.22 a | 57.09 ± 1.33 b |
| a* | −7.78 ± 0.02 d | −6.32 ± 0.38 c | 2.21 ± 0.50 b | 8.01 ± 0.58 a |
| b* | 20.64 ± 0.47 a | 21.87 ± 0.96 a | 16.33 ± 0.97 b | 19.88 ± 0.73 a |
| Chroma (C*) | 22.06 ± 0.43 a | 22.77 ± 0.91 a | 16.48 ± 0.97 b | 21.43 ± 0.90 a |
| Hue angle (h) | 110.66 ± 0.48 a | 106.13 ± 1.21 b | 82.29 ± 1.66 c | 68.07 ± 0.71 d |
| Parameter | Yerba-Mate Leaves (mg/g) | Beverage (mg/mL) | ||
|---|---|---|---|---|
| PL | NT | PL | NT | |
| 5-CQA | 19.323 ± 0.243 a | 17.939 ± 0.481 b | 0.185 ± 0.002 c | 0.251 ± 0.008 c |
| Caffeic acid | 0.137 ± 0.006 a | nd | 0.009 ± 0.000 b | 0.005 ± 0.000 b |
| Rutin | 8.615 ± 0.196 a | 6.081 ± 0.141 b | 0.052 ± 0.001 c | 0.051 ± 0.001 c |
| ABTS * | 424.66 ± 0.03 b | 485.47 ± 0.03 a | 5.63 ± 0.02 d | 8.18 ± 0.02 c |
| DPPH (IC50) | 39.72 ± 0.01 a | 38.64 ± 0.01 b | 147.06 ± 0.02 c | 92.83 ± 0.01 d |
| Parameter | Yerba-Mate Leaves (mg/g) | Beverage (mg/mL) | ||
|---|---|---|---|---|
| PL | NT | PL | NT | |
| Theobromine | 0.043 ± 0.008 b | 0.484 ± 0.036 a | <QL | <QL |
| Theophylline | nd | nd | nd | nd |
| Caffeine | 1.642 ± 0.183 b | 5.048 ± 0.221 a | 0.049 ± 0.002 c | 0.183 ± 0.003 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frizon, C.N.T.; Perussello, C.A.; Sturion, J.A.; Hoffmann-Ribani, R. Novel Beverages of Yerba-Mate and Soy: Bioactive Compounds and Functional Properties. Beverages 2018, 4, 21. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages4010021
Frizon CNT, Perussello CA, Sturion JA, Hoffmann-Ribani R. Novel Beverages of Yerba-Mate and Soy: Bioactive Compounds and Functional Properties. Beverages. 2018; 4(1):21. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages4010021
Chicago/Turabian StyleFrizon, Cátia Nara Tobaldini, Camila Augusto Perussello, José Alfredo Sturion, and Rosemary Hoffmann-Ribani. 2018. "Novel Beverages of Yerba-Mate and Soy: Bioactive Compounds and Functional Properties" Beverages 4, no. 1: 21. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages4010021