De Novo Valve Tissue Morphology Following Bioscaffold Mitral Valve Replacement in a Juvenile Non-Human Primate Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vivo Pilot Assessment of PSIS Mitral Valve
2.2. Surgical Preparation and Procedure for PSIS Mitral Valve Implantations
2.3. Histological and Immunostaining of Explanted PSIS Mitral Valves for Assessment of Somatic Growth and Extracellular Content
2.4. Spatial Intensity Mapping of Explanted PSIS Mitral Valves for Assessment of Percent Extracellular Matrix Content
2.5. Statistical Analysis
3. Results
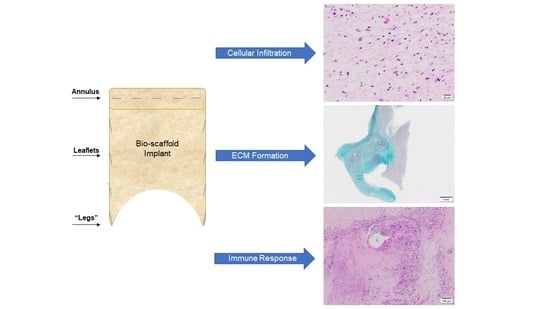
3.1. Microstructure and Phenotype of De Novo Valve Tissues on Explanted PSIS Mitral Valves
3.1.1. Cellular Infiltration and Morphology of Explanted Native and PSIS Mitral Valve Leaflets
3.1.2. Phenotype of Native and Explanted PSIS Mitral Valve Leaflets
3.2. Extracellular Matrix Assessment via Spatial Intensity Quantifications on Explanted Mitral Valves
3.2.1. Extracellular Matrix Assessment via Spatial Intensity Quantifications of Explanted Native and PSIS Mitral Valve Leaflet
3.2.2. Extracellular Matrix Assessment via Spatial Intensity Quantifications of Explanted PSIS Mitral Valve Annulus
3.2.3. Extracellular Matrix Assessment via Spatial Intensity Quantifications of Explanted PSIS Mitral Valve “Legs”
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, A.K.; Weiner, M.M.; Feinman, J.W.; Bhatt, H.V.; Fritz, A.V.; Townsley, M.M.; Sharma, A.; Stawiarski, K.; Patel, S.J.; Zhou, E.Y.; et al. The Year in Cardiothoracic and Vascular Anesthesia: Selected Highlights from 2020. J. Cardiothorac. Vasc. Anesth. 2021, 35, 993–1005. [Google Scholar] [CrossRef]
- Hinton, R.B.; Yutzey, K.E. Heart valve structure and function in development and disease. Annu. Rev. Physiol. 2011, 73, 29–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henaine, R.; Roubertie, F.; Vergnat, M.; Ninet, J. Valve replacement in children: A challenge for a whole life. Arch. Cardiovasc. Dis. 2012, 105, 517–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blitzer, D.; Herrmann, J.L.; Brown, J.W. Pulmonary Autograft Mitral Valve Replacement (Ross II): Long-Term Follow-Up of a US Center. World J. Pediatr. Congenit. Hear. Surg. 2018, 9, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.L.F.; van Geemen, D.; van den Bogaerdt, A.J.; Oomens, C.W.J.; Bouten, C.V.C.; Baaijens, F.P.T. Mechanics of the pulmonary valve in the aortic position. J. Mech. Behav. Biomed. Mater. 2014, 29, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.Y.; Duan, B.; Butcher, J.T. Bioprinting of Cardiac Tissues. In Essentials of 3D Biofabrication and Translation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 351–370. [Google Scholar]
- Sandusky, G.E., Jr.; Badylak, S.F.; Morff, R.J.; Johnson, W.D.; Lantz, G. Histologic findings after in vivo placement of small intestine submucosal vascular grafts and saphenous vein grafts in the carotid artery in dogs. Am. J. Pathol. 1992, 140, 317. [Google Scholar]
- Robotin-Johnson, M.C.; Swanson, P.E.; Johnson, D.C.; Schuessler, R.B.; Cox, J.L. An experimental model of small intestinal submucosa as a growing vascular graft. J. Thorac. Cardiovasc. Surg. 1998, 116, 805–811. [Google Scholar] [CrossRef] [Green Version]
- Matheny, R.G.; Hutchison, M.L.; Dryden, P.E.; Hiles, M.D.; Shaar, C.J. Porcine small intestine submucosa as a pulmonary valve leaflet substitute. J. Heart Valve Dis. 2000, 9, 769–774, discussion 774. [Google Scholar]
- Rosen, M.; Roselli, E.E.; Faber, C.; Ratliff, N.B.; Ponsky, J.L.; Smedira, N.G. Small intestinal submucosa intracardiac patch: An experimental study. Surg. Innov. 2005, 12, 227–231. [Google Scholar] [CrossRef]
- Ruiz, C.E.; Iemura, M.; Medie, S.; Varga, P.; Van Alstine, W.G.; Mack, S.; DeLigio, A.; Fearnot, N.; Beier, U.; Pavcnik, D.; et al. Transcatheter placement of a low-profile biodegradable pulmonary valve made of small intestinal submucosa: A long-term study in a swine model. J. Thorac. Cardiovasc. Surg. 2005, 130, 477.e1–477.e9. [Google Scholar] [CrossRef] [Green Version]
- White, J.K.; Agnihotri, A.K.; Titus, J.S.; Torchiana, D.F. A stentless trileaflet valve from a sheet of decellularized porcine small intestinal submucosa. Ann. Thorac. Surg. 2005, 80, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, K.; Geyik, S.; Pavcnik, D.; Uchida, B.T.; Corless, C.L.; Hartley, D.E.; Goktay, A.; Correa, L.O.; Timmermans, H.; Hodde, J.P.; et al. Comparison of the endothelialization of small intestinal submucosa, dacron, and expanded polytetrafluoroethylene suspended in the thoracoabdominal aorta in sheep. J. Vasc. Interv. Radiol. 2006, 17, 873–882. [Google Scholar] [CrossRef]
- Pavčnik, D.; Obermiller, J.; Uchida, B.T.; Van Alstine, W.; Edwards, J.M.; Landry, G.J.; Kaufman, J.A.; Keller, F.S.; Rösch, J. Angiographic evaluation of carotid artery grafting with prefabricated small-diameter, small-intestinal submucosa grafts in sheep. Cardiovasc. Interv. Radiol. 2009, 32, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, A.; Tyralla, M.; Ladhoff, J.; Schleicher, M.; Stock, U.A.; Volk, H.-D.; Seifert, M. Human immune responses to porcine xenogeneic matrices and their extracellular matrix constituents in vitro. Biomaterials 2010, 31, 3793–3803. [Google Scholar] [CrossRef] [PubMed]
- Scholl, F.G.; Boucek, M.M.; Chan, K.-C.; Valdes-Cruz, L.; Perryman, R. Preliminary experience with cardiac reconstruction using decellularized porcine extracellular matrix scaffold: Human applications in congenital heart disease. World J. Pediatr. Congenit. Hear. Surg. 2010, 1, 132–136. [Google Scholar] [CrossRef]
- Gilbert, C.L.; Gnanapragasam, J.; Benhaggen, R.; Novick, W.M. Novel use of extracellular matrix graft for creation of pulmonary valved conduit. World J. Pediatr. Congenit. Hear. Surg. 2011, 2, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Quarti, A.; Nardone, S.; Colaneri, M.; Santoro, G.; Pozzi, M. Preliminary experience in the use of an extracellular matrix to repair congenital heart diseases. Interact. Cardiovasc. Thorac. Surg. 2011, 13, 569–572. [Google Scholar] [CrossRef] [Green Version]
- Boni, L.; Chalajour, F.; Sasaki, T.; Snyder, R.L.; Boyd, W.D.; Riemer, R.K.; Reddy, V.M. Reconstruction of pulmonary artery with porcine small intestinal submucosa in a lamb surgical model: Viability and growth potential. J. Thorac. Cardiovasc. Surg. 2012, 144, 963–969.e1. [Google Scholar] [CrossRef] [Green Version]
- Fallon, A.; Goodchild, T.; Gilbert, C.; Matheny, R. A Pulmonary Valved Conduit of Porcine SIS Remodels into Native Tissue in an Ovine Model. In Proceedings of the 5th Biennial Conference on Heart Valve Biology and Tissue Engineering, Mykonos, Greece, 18–20 May 2012; Hamad bin Khalifa University Press (HBKU Press): Doha, Qatar, 2012. [Google Scholar]
- Poulin, F.; Horlick, E.; David, T.; Woo, A.; Thavendiranathan, P. 3-Dimensional transesophageal echocardiography–guided closure of a gerbode shunt due to cormatrix patch dehiscence. J. Am. Coll. Cardiol. 2013, 62, e5. [Google Scholar] [CrossRef] [Green Version]
- Yanagawa, B.; Rao, V.; Yau, T.M.; Cusimano, R.J. Potential myocardial regeneration with CorMatrix ECM: A case report. J. Thorac. Cardiovasc. Surg. 2014, 147, e41–e43. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, A.H.; Nathan, M.; Emani, S.; Baird, C.; del Nido, P.J.; Gauvreau, K.; Harris, M.; Sanders, S.P.; Padera, R.F. Preliminary experience with porcine intestinal submucosa (CorMatrix) for valve reconstruction in congenital heart disease: Histologic evaluation of explanted valves. J. Thorac. Cardiovasc. Surg. 2014, 148, 2216–2225.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bibevski, S.; Scholl, F.G. Feasibility and early effectiveness of a custom, hand-made systemic atrioventricular valve using porcine extracellular matrix (CorMatrix) in a 4-month-old infant. Ann. Thorac. Surg. 2015, 99, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Padalino, M.A.; Quarti, A.; Angeli, E.; Frigo, A.C.; Vida, V.L.; Pozzi, M.; Gargiulo, G.; Stellin, G. Early and mid-term clinical experience with extracellular matrix scaffold for congenital cardiac and vascular reconstructive surgery: A multicentric Italian study. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Soucy, K.G.; Smith, E.F.; Monreal, G.; Rokosh, G.; Keller, B.B.; Yuan, F.; Matheny, R.G.; Fallon, A.M.; Lewis, B.C.; Sherwood, L.C.; et al. Feasibility study of particulate extracellular matrix (P-ECM) and left ventricular assist device (HVAD) therapy in chronic ischemic heart failure bovine model. Asaio J. 2015, 61, 161–169. [Google Scholar] [CrossRef]
- Mosala Nezhad, Z.; Poncelet, A.; De Kerchove, L.; Gianello, P.; Fervaille, C.; El Khoury, G. Small intestinal submucosa extracellular matrix (CorMatrix®) in cardiovascular surgery: A systematic review. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 839–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, J.S.; Fishbein, M.C.; Reemtsen, B. Histologic examination of decellularized porcine intestinal submucosa extracellular matrix (CorMatrix) in pediatric congenital heart surgery. Cardiovasc. Pathol. 2016, 25, 12–17. [Google Scholar] [CrossRef]
- Bibevski, S.; Levy, A.; Scholl, F.G. Mitral valve replacement using a handmade construct in an infant. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 639–640. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, B.; Hernandez, L.; Bibevski, S.; Scholl, F.; Brehier, V.; Casares, M.; Bibevski, J.; Rivas, K.; Morales, P.; Wagner, J.; et al. Recapitulation of human bio-scaffold mitral valve growth in the baboon model. Circulation 2018, 138, A11348. [Google Scholar]
- Gonzalez, B.; Perez, M.G.; Mirza, A.; Scholl, F.; Bibevski, S.; Wagner, K.R.; Bibevski, J.; Hernandez, L.E.; Ladich, E.; Brehier, V.; et al. Extracellular Matrix Quantification of Fully Regenerated Neochorade After Bio-scaffold Mitral Valve Implantation in a Juvenile Non-human Primate Model. Circulation 2020, 142, A14888. [Google Scholar] [CrossRef]
- Gonzalez, B.A.; Pour Issa, E.; Mankame, O.V.; Bustillos, J.; Cuellar, A.; Rodriguez, A.J.; Scholl, F.; Bibevski, S.; Hernandez, L.; Brehier, V.; et al. Porcine small intestinal submucosa mitral valve material responses support acute somatic growth. Tissue Eng. Part A 2020, 26, 475–489. [Google Scholar] [CrossRef]
- Badylak, S.; Geddes, L.; Obermiller, J. Extracellular matrix for myocardial repair. Hear. Surg. Forum 2003, 6, 20. [Google Scholar] [CrossRef]
- Slaughter, M.S.; Soucy, K.G.; Matheny, R.G.; Lewis, B.C.; Hennick, M.F.; Choi, Y.; Monreal, G.; Sobieski, M.A.; Giridharan, G.A.; Koenig, S.C. Development of an extracellular matrix delivery system for effective intramyocardial injection in ischemic tissue. ASAIO J. 2014, 60, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Pavcnik, D.; Uchida, B.T.; Timmermans, H.A.; Corless, C.L.; O’Hara, M.; Toyota, N.; Moneta, G.L.; Keller, F.S.; Rösch, J. Percutaneous bioprosthetic venous valve: A long-term study in sheep. J. Vasc. Surg. 2002, 35, 598–602. [Google Scholar] [CrossRef] [Green Version]
- Witt, R.G.; Raff, G.; Van Gundy, J.; Rodgers-Ohlau, M.; Si, M.-S. Short-term experience of porcine small intestinal submucosa patches in paediatric cardiovascular surgery. Eur. J. Cardio Thorac. Surg. 2013, 44, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Gerdisch, M.W.; Boyd, W.D.; Harlan, J.L.; Richardson, J.B.; Flack, J.E.; Palafox, B.A.; Johnson, W.E.; Sun, B.; Lee, R.; Guy, T.S.; et al. Early experience treating tricuspid valve endocarditis with a novel extracellular matrix cylinder reconstruction. J. Thorac. Cardiovasc. Surg. 2014, 148, 3042–3048. [Google Scholar] [CrossRef] [Green Version]
- Zafar, F.; Hinton, R.B.; Moore, R.A.; Baker, R.S.; Bryant, R.; Narmoneva, D.A.; Taylor, M.D.; Morales, D.L. Physiological growth, remodeling potential, and preserved function of a novel bioprosthetic tricuspid valve: Tubular bioprosthesis made of small intestinal submucosa-derived extracellular matrix. J. Am. Coll. Cardiol. 2015, 66, 877–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rijswijk, J.W.; Talacua, H.; Mulder, K.; van Hout, G.P.; Bouten, C.V.; Gründeman, P.F.; Kluin, J. Failure of decellularized porcine small intestinal submucosa as a heart valved conduit. J. Thorac. Cardiovasc. Surg. 2020, 160, e201–e215. [Google Scholar] [CrossRef]
- Gonzalez, B.A. Bioscaffold Valve with and without Mechanically Conditioned Stem Cells for the Treatment of Critical Mitral Valve Diseases in the Young. Ph.D. Thesis, Florida International University, Miami, FL, USA, 2020. unpulished. [Google Scholar]
- VeDepo, M.C.; Detamore, M.S.; Hopkins, R.A.; Converse, G.L. Recellularization of decellularized heart valves: Progress toward the tissue-engineered heart valve. J. Tissue Eng. 2017, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Vigne, J.; Bay, S.; Aid-Launais, R.; Pariscoat, G.; Rucher, G.; Sénémaud, J.; Truffier, A.; Anizan, N.; Even, G.; Ganneau, C.; et al. Cleaved CD31 as a target for in vivo molecular imaging of inflammation. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Tian, X.Y. The Role of Macrophages in Vascular Repair and Regeneration after Ischemic Injury. Int. J. Mol. Sci. 2020, 21, 6328. [Google Scholar] [CrossRef]
- Cutie, S.; Huang, G.N. Vertebrate Cardiac Regeneration: Evolutionary and Developmental Perspectives. Cell Regener. 2021, 10, 6. [Google Scholar]
- Moreira, R.; Velz, T.; Alves, N.; Gesche, V.N.; Malischewski, A.; Schmitz-Rode, T.; Frese, J.; Jockenhoevel, S.; Mela, P. Tissue-engineered heart valve with a tubular leaflet design for minimally invasive transcatheter implantation. Tissue Eng. Part C Methods 2015, 21, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Derm. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Baasanjav, S.; Al-Gazali, L.; Hashiguchi, T.; Mizumoto, S.; Fischer, B.; Horn, D.; Seelow, D.; Ali, B.R.; Aziz, S.A.; Langer, R.; et al. Faulty initiation of proteoglycan synthesis causes cardiac and joint defects. Am. J. Hum. Genet. 2011, 89, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Ashley, E.A.; Niebauer, J. Cardiology Explained; Remedica: London, UK, 2004. [Google Scholar]
- Eslami, M.; Javadi, G.; Agdami, N.; Shokrgozar, M.A.; Eslami, M.; Javadi, G.; Agdami, N.; Shokrgozar, M.A. Expression of COLLAGEN 1 and ELASTIN genes in mitral valvular interstitial cells within microfiber reinforced hydrogel. Cell J. 2015, 17, 478–488. [Google Scholar] [PubMed]














| Baboon Valve Type | Area | % Collagen | % Elastin | % Proteoglycans | % Fibrin |
|---|---|---|---|---|---|
| Native Mitral Valve | Leaflet | 41% ± 0.07 | 12% ± 0.02 | 46% ± 0.07 | - |
| 3-Month PSIS Explant | Leaflet | 45% ± 0.11 | 7% ± 0.02 | 24% ± 0.21 | - |
| 11-Month PSIS Explant | Leaflet | 40% ± 0.07 | 11% ± 0.02 | 48% ± 0.06 | 1% ± 0.00 |
| 20-Month PSIS Explant | Leaflet | 49% ± 0.06 | 9% ± 0.01 | 40% ± 0.04 | 3% ± 0.01 |
| Baboon Valve Type | Area | % Collagen | % Elastin | % Proteoglycans | % Fibrin |
|---|---|---|---|---|---|
| 3-Month PSIS Explant | Annulus | 61% | 12% | 5% * + | - |
| 11-Month PSIS Explant | Annulus | 41% ± 0.04 | 14% ± 0.02 | 44% ± 0.03 * | 1% ± 0.00 |
| 20-Month PSIS Explant | Annulus | 52% ± 0.07 | 6% ± 0.02 | 38% ± 0.05 + | 4% ± 0.03 |
| Baboon Valve Tye | Area | % Collagen | % Elastin | % Proteoglycans | % Fibrin |
|---|---|---|---|---|---|
| 11-Month PSIS Explant | NC/PM | 50% ± 0.06 | 11% ± 0.0 | 37% ± 0.07 | 2% ± 0.01 |
| 20-Month PSIS Explant | NC/PM | 43% ±0.06 | 9% ± 0.01 | 47% ± 0.06 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez, B.A.; Perez Gonzalez, M.; Scholl, F.; Bibevski, S.; Ladich, E.; Bibevski, J.; Morales, P.; Lopez, J.; Casares, M.; Brehier, V.; et al. De Novo Valve Tissue Morphology Following Bioscaffold Mitral Valve Replacement in a Juvenile Non-Human Primate Model. Bioengineering 2021, 8, 100. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering8070100
Gonzalez BA, Perez Gonzalez M, Scholl F, Bibevski S, Ladich E, Bibevski J, Morales P, Lopez J, Casares M, Brehier V, et al. De Novo Valve Tissue Morphology Following Bioscaffold Mitral Valve Replacement in a Juvenile Non-Human Primate Model. Bioengineering. 2021; 8(7):100. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering8070100
Chicago/Turabian StyleGonzalez, Brittany A., Marcos Perez Gonzalez, Frank Scholl, Steven Bibevski, Elena Ladich, Jennifer Bibevski, Pablo Morales, Jesus Lopez, Mike Casares, Vincent Brehier, and et al. 2021. "De Novo Valve Tissue Morphology Following Bioscaffold Mitral Valve Replacement in a Juvenile Non-Human Primate Model" Bioengineering 8, no. 7: 100. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering8070100