Hide-and-Seek with Tiny Neotenic Beetles in One of the Hottest Biodiversity Hotspots: Towards an Understanding of the Real Diversity of Jurasaidae (Coleoptera: Elateroidea) in the Brazilian Atlantic Forest
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Systematics
3.1.1. New Species
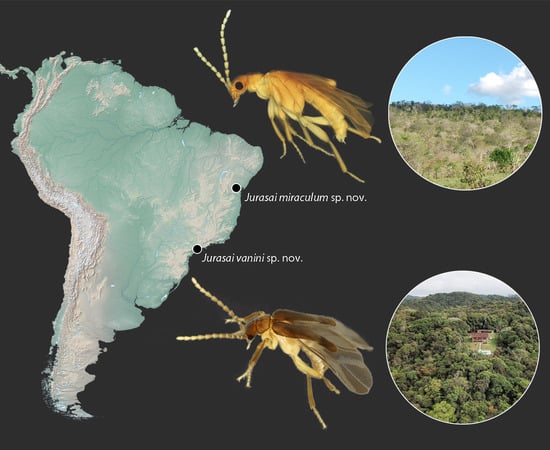
- Jurasai miraculum sp. nov.
- Jurasai vanini sp. nov.
3.1.2. New Distributional Record and Remarks
3.2. Identification Key to Genera and Species of Jurasaidae
| 1. Pronotum with lateral carina; elytra long, almost completely covering the hind wings, parallel-sided, median edges contiguous to apex, apices flat; tarsomere IV deeply notched |
| Tujamita plenalatum Rosa et al., 2020 |
| –. Pronotum without lateral carina (Figure 2b, Figure 4b and Figure 6b); elytra short, exposing apical third of hind wing, with median edges separated and divergent apicad, lateral edges sinuate, apices swollen (Figure 1, Figure 3 and Figure 5); tarsomere IV truncate |
| 2 (Jurasai Rosa et al., 2020) |
| 2. Elytra strongly tapered on posterior 2/3; sternite VIII partly exposed |
| J. itajubense Rosa et al., 2020 |
| –. Elytra weakly tapered on posterior 1/3 (Figure 1, Figure 3 and Figure 5); sternite VIII concealed |
| 3 |
| 3. Maxillary palpus four-segmented (Figure 2a,b); antenna short, reaching basal third of elytra; pronotum widest at middle (Figure 2e); phallus 1.4 times longer than parameres (Figure 2f–j) |
| J. miraculum sp. nov. |
| –. Maxillary palpus five-segmented (Figure 4b and Figure 6b); antenna long, reaching half of elytra; pronotum widest at anterior third (Figure 4d and Figure 6f); phallus as long as parameres (Figure 4j–m and Figure 6g–i) |
| 4 |
| 4. Labrum truncate to slightly emarginate (Figure 6a,c–e); phallus tapered (Figure 6g,h) |
| J. digitusdei Rosa et al., 2020 |
| –. Labrum deeply emarginate (Figure 4c,e); phallus cylindrical with apical flap (Figure 4j–m) |
| J. vanini sp. nov. |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gould, S.J. Ontogeny and Phylogeny; Harvard University Press: Cambridge, MA, USA, 1977; pp. 1–520. [Google Scholar]
- Cicero, J.M. Ontophylogenetics of cantharoid larviforms (Coleoptera: Cantharoidea). Coleopt. Bull. 1988, 42, 105–151. [Google Scholar]
- McMahon, D.P.; Hayward, A. Why grow up? A perspective on insect strategies to avoid metamorphosis. Ecol. Entomol. 2016, 41, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Johnston, M.A.; Gimmel, M.L. Review of North American Dascillidae (Coleoptera: Dascilloidea), with descriptions of dramatic female wing reduction. Coleopt. Bull. 2020, 74, 731–757. [Google Scholar] [CrossRef]
- Bocakova, M.; Bocak, L.; Hunt, T.; Teräväinen, M.; Vogler, A.P. Molecular phylogenetics of Elateriformia (Coleoptera): Evolution of bioluminescence and neoteny. Cladistics 2007, 23, 477–496. [Google Scholar] [CrossRef]
- Bocak, L.; Bocakova, M.; Hunt, T.; Vogler, A.P. Multiple ancient origins of neoteny in Lycidae (Coleoptera): Consequences for ecology and macroevolution. Proc. R. Soc. B 2008, 275, 2015–2023. [Google Scholar] [CrossRef] [Green Version]
- South, A.; Stanger-Hall, K.; Jeng, M.-L.; Lewis, S.M. Correlated evolution of female neoteny and flightlessness with male spermatophore production in fireflies (Coleopteta: Lampyridae). Evolution 2010, 65, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Kundrata, R.; Bocak, L. Molecular phylogeny reveals the gradual evolutionary transition to soft-bodiedness in click-beetles and identifies Sub-Saharan Africa as a cradle of diversity for Drilini (Coleoptera: Elateridae). Zool. J. Linn. Soc. 2019, 187, 413–452. [Google Scholar] [CrossRef]
- Ferreira, V.S.; Keller, O.; Branham, M.A. Multilocus phylogeny support the nonbioluminescent firefly Chespirito as a new subfamily in the Lampyridae (Coleoptera: Elateroidea). Insect Syst. Divers. 2020, 4, 2. [Google Scholar] [CrossRef]
- Crowson, R.A. A review of the classification of Cantharoidea (Coleoptera), with the definition of two new families Cneoglossidae and Omethidae. Rev. Univ. Madrid 1972, 21, 35–77. [Google Scholar]
- Lawrence, J.F. Rhinorhipidae, a new beetle family from Australia, with comments on the phylogeny of the Elateriformia. Invertebr. Taxon. 1988, 2, 1–53. [Google Scholar] [CrossRef]
- Kundrata, R.; Bocakova, M.; Bocak, L. The comprehensive phylogeny of the superfamily Elateroidea (Coleoptera: Elateriformia). Mol. Phylogenet. Evol. 2014, 76, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Bocak, L.; Kundrata, R.; Andújar Fernández, C.; Vogler, A.P. The discovery of Iberobaeniidae (Coleoptera: Elateroidea), a new family of beetles from Spain, with immatures detected by environmental DNA sequencing. Proc. R. Soc. B 2016, 283, 20152350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malohlava, V.; Bocak, L. Evidence of extreme habitat stability in a Southeast Asian biodiversity hotspot based on the evolutionary analysis of neotenic net-winged beetles. Mol. Ecol. 2010, 19, 4800–4811. [Google Scholar] [CrossRef]
- Bray, T.C.; Bocak, L. Slowly dispersing neotenic beetles can speciate on a penny coin and generate space-limited diversity in the tropical mountains. Sci. Rep. 2016, 6, 33579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundrata, R.; Bocak, L. Taxonomic review of Drilini (Elateridae: Agrypninae) in Cameroon reveals high morphological diversity, including the discovery of five new genera. Insect Syst. Evol. 2017, 48, 441–492. [Google Scholar] [CrossRef]
- Janisova, K.; Bocakova, M. Revision of the subfamily Ototretinae (Coleoptera: Lampyridae). Zool. Anz. 2013, 252, 1–19. [Google Scholar] [CrossRef]
- Ferreira, V.S.; Keller, O.; Branham, M.A.; Ivie, M.A. Molecular data support the placement of the enigmatic Cheguevaria as a subfamily of Lampyridae (Insecta: Coleoptera). Zool. J. Linn. Soc. 2019, 187, 1253–1258. [Google Scholar] [CrossRef]
- Ferreira, V.S.; Silveira, L.F.L. A new suspected paedomorphic genus of net-winged beetles from the Atlantic Rainforest (Coleoptera, Elateroidea, Lycidae). Pap. Avulsos Zool. 2020, 60, e202060(s.i.).35. [Google Scholar] [CrossRef]
- Kundrata, R.; Baena, M.; Bocak, L. Iberobaenia andujari sp. nov., the third species of Iberobaeniidae (Coleoptera: Elateroidea) from southern Spain. Ann Zool. 2017, 67, 121–129. [Google Scholar] [CrossRef]
- Rosa, S.P.; Costa, C.; Kramp, K.; Kundrata, R. Hidden diversity in the Brazilian Atlantic rainforest: The discovery of Jurasaidae, a new beetle family (Coleoptera, Elateroidea) with neotenic females. Sci. Rep. 2020, 10, 1544. [Google Scholar] [CrossRef] [Green Version]
- Rosa, S.P.; Costa, C.; Kramp, K.; Kundrata, R. Author Correction: Hidden diversity in the Brazilian Atlantic rainforest: The discovery of Jurasaidae, a new beetle family (Coleoptera, Elateroidea) with neotenic females. Sci. Rep. 2020, 10, 3769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brancucci, M. Morphologie comparée, évolution et systématique des Cantharidae (Insecta: Coleoptera). Entomol. Basil. 1980, 5, 215–388. [Google Scholar]
- Tabarelli, M.; Aguiar, A.V.; Ribeiro, M.C.; Metzger, J.P.; Peres, C.A. Prospects for biodiversity conservation in the Atlantic Forest: Lessons from aging human-modified landscapes. Biol. Conserv. 2010, 143, 2328–2340. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Martensen, A.C.; Metzger, J.P.; Tabarelli, M.; Scarano, F.; Fortin, M.J. The Brazilian Atlantic Forest: A shrinking biodiversity hotspot. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Zachos, F., Habel, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 405–434. [Google Scholar]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global biodiversity conservation: The critical role of hotspots. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Zachos, F.E., Habel, J.C., Eds.; Springer: Berlin, Heidelberg/Germany, 2011; pp. 3–22. [Google Scholar]
- Joly, C.A.; Metzger, J.P.; Tabarelli, M. Experiences from the Brazilian Atlantic Forest: Ecological findings and conservation initiatives. New Phytol. 2014, 204, 459–473. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.M.C.; Pinto, L.P.; Hirota, M.; Bedê, L.; Tabarelli, M. Conservação da Mata Atlântica brasileira—Um balanço dos últimos dez anos. In Metamorfoses florestais: Culturas, ecologias e as transformações históricas da Mata Atlântica; Cabral, D.C., Bustamante, A.G., Eds.; Prismas: Curitiba, Brazil, 2016; pp. 435–458. [Google Scholar]
- Rezende, C.L.; Scarano, F.R.; Assad, E.D.; Joly, C.A.; Metzger, J.P.; Strassburg, B.B.N.; Tabarelli, M.; Fonseca, G.A.; Mittermeier, R.A. From hotspot to hopespot: An opportunity for the Brazilian Atlantic Forest. Perspect. Ecol. Conserv. 2018, 16, 208–214. [Google Scholar] [CrossRef]
- Morellato, L.P.C.; Haddad, C.F.B. Introduction: The Brazilian Atlantic Forest. Biotropica 2000, 32, 786–792. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Metzger, J.P.; Martensen, A.C.; Ponzoni, F.J.; Hirota, M.M. The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 2009, 142, 1141–1153. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef] [Green Version]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Laurance, W.F. Conserving the hottest of the hotspots. Biol. Conserv. 2009, 142, 1137. [Google Scholar] [CrossRef]
- Almeida, F.F.M.; Carneiro, C.D.R. Origem e evolução da Serra do Mar. Rev. Bras. Geociênc. 1998, 28, 135–150. [Google Scholar] [CrossRef]
- Instituto Florestal. Parque Estadual da Serra do Mar: Plano de Manejo; Secretaria do Meio Ambiente, Instituto Florestal, Divisão de Reservas e Parques Estaduais: São Paulo, Brazil, 2008; pp. 1–441. [Google Scholar]
- França, F.; Melo, E.; Santos, C.C. Flora de inselbergs da região de Milagres, Bahia, Brasil: I—Caracterização da vegetação e lista de espécies de dois inselbergs. Sitientibus 1997, 17, 163–184. [Google Scholar]
- Prado, D.E. As Caatingas da América do Sul. In Ecologia e Conservação da Caatinga; Leal, I.R., Tabarelli, M., Silva, J.M.C., Eds.; Editora Universitária da UFPE: Recife, Brazil, 2003; pp. 3–73. [Google Scholar]
- Leal, I.R.; Tabarelli, M.; Silva, J.M.C. Ecologia e Conservação da Caatinga; Editora Universitária da UFPE: Recife, Brazil, 2003; pp. 1–822. [Google Scholar]
- Leal, I.R.; Silva, J.M.C.; Tabarelli, M.; Lacher, T.E., Jr. Changing the course of biodiversity conservation in the caatinga of northeastern Brazil. Conserv. Biol. 2005, 19, 701–703. [Google Scholar] [CrossRef]
- Santos, J.C.; Leal, I.R.; Almeida-Cortez, J.S.; Fernandes, G.W.; Tabarelli, M. Caatinga: The scientific negligence experienced by a Dry Tropical Forest. Trop. Conserv. Sci. 2011, 4, 276–286. [Google Scholar] [CrossRef]
- Vieira, F.A.; Novaes, R.M.L.; Fajardo, C.G.; Santos, R.M.; Almeida, H.S.; Carvalho, D.; Lovato, M.B. Holocene southward expansion in seasonally dry tropical forests in South America: Phylogeography of Ficus bonijesulapensis (Moraceae). Bot. J. Linn. Soc. 2015, 177, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, D.B.O.S.; Queiroz, L.P. Caatinga no contexto de uma metacomunidade: Evidências da biogeografia, padrões filogenéticos e abundância de espécies em Leguminosas. In Biogeografia da América do Sul: Padrões e processos; Carvalho, C.J.B., Almeida, E.A.B., Eds.; Roca: São Paulo, Brazil, 2010; pp. 241–260. [Google Scholar]
- Pennington, R.T.; Prado, D.A.; Pendry, C. Neotropical seasonally dry forests and quaternary vegetation changes. J. Biogeogr. 2000, 27, 261–273. [Google Scholar] [CrossRef]
- Tabarelli, M.; Vicente, A. Conhecimento sobre plantas lenhosas da Caatinga: Lacunas geográficas e ecológicas. In Biodiversidade da Caatinga: Áreas e Ações Prioritárias para a Conservação; Silva, J.M.C., Tabarelli, M., Fonseca, M.T., Lins, L.V., Eds.; Ministério do Meio Ambiente: Brasília, Brazil, 2004; pp. 101–111. [Google Scholar]
- Ulysséa, M.A.; Brandão, C.R.F. Ant species (Hymenoptera, Formicidae) from the seasonally dry tropical forest of northeastern Brazil: A compilation from field surveys in Bahia and literature records. Rev. Bras. Entomol. 2013, 57, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Bravo, F.; Calor, A. Artrópodes do Semiárido: Biodiversidade e Conservação; Printmídia: Feira de Santana, Brazil, 2014; pp. 1–298. [Google Scholar]
- Velloso, A.L.; Sampaio, E.V.S.B.; Pareyn, F.G.C. Ecorregiões Propostas para o Bioma Caatinga; Associação Plantas do Nordeste, Instituto de Conservação Ambiental: Recife, Brazil, 2002; pp. 1–80. [Google Scholar]
- Ministério do Meio Ambiente. Áreas Prioritárias para a Conservação, Utilização Sustentável e Repartição de Benefícios da Biodiversidade Brasileira ou Áreas Prioritárias para a Biodiversidade. Portaria nº 463, de 18 de Dezembro de 2018. Available online: http://areasprioritarias.mma.gov.br/2-atualizacao-das-areas-prioritarias (accessed on 18 January 2021).
- Bocak, L.; Matsuda, K. Review of the immature stages of the family Lycidae (Insecta: Coleoptera). J. Nat. Hist. 2003, 37, 1463–1507. [Google Scholar] [CrossRef]
- Werneck, F.P.; Costa, G.C.; Colli, G.R.; Prado, D.E.; Sites, J.W., Jr. Revisiting the historical distribution of Seasonally Dry Tropical Forests: New insights based on palaeodistribution modelling and palynological evidence. Glob. Ecol. Biogeogr. 2011, 20, 272–288. [Google Scholar] [CrossRef]
- Wilson, E.O. Biodiversity research requires more boots on the ground. Nat. Ecol. Evol. 2017, 1, 1590. [Google Scholar] [CrossRef]
- Marinoni, R.C.; Dutra, R.R.C. Famílias de Coleoptera capturadas com armadilha Malaise em oito localidades do Estado do Paraná, Brasil. Diversidades alfa e beta. Rev. Brasil Zool. 1997, 14, 751–770. [Google Scholar] [CrossRef] [Green Version]
- Cancello, E.M.; Silva, R.R.; Vasconcellos, A.; Reis, Y.T.; Oliveira, L.M. Latitudinal variation in termite species richness and abundance along the Brazilian Atlantic Forest hotspot. Biotropica 2014, 46, 441–450. [Google Scholar] [CrossRef]
- Silva, R.R.; Brandão, C.R.F. Ecosystem-wide morphological structure of leaf-litter ant communities along a tropical latitudinal gradient. PLoS ONE 2014, 9, e93049. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.M.; Schlindwein, M.N.; Viviani, V.R. Survey of bioluminescent Coleoptera in the Atlantic rain forest of Serra da Paranapiacaba in São Paulo State (Brazil). Biota Neotrop. 2016, 16, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Silveira, L.F.L.; Khattar, G.; Vaz, S.; Wilson, V.A.; Souto, P.M.; Mermudes, J.R.M.; Stanger-Hall, K.F.; Macedo, M.V.; Monteiro, R.F. Natural history of the fireflies of the Serra dos Órgãos mountain range (Brazil: Rio de Janeiro)—One of the ‘hottest’ firefly spots on Earth, with a key to genera (Coleoptera: Lampyridae). J. Nat. Hist. 2020, 54, 275–308. [Google Scholar] [CrossRef]
- Azevedo, C.O.; Molin, A.D.; Penteado-Dias, A.; Macedo, A.C.C.; Rodriguez, V.A.; Dias, B.Z.K.; Waichert, C.; Aquino, D.; Smith, D.R.; Shimbori, E.M.; et al. Checklist of the genera of Hymenoptera (Insecta) from Espírito Santo state, Brazil. Bol. Mus. Biol. Mello Leitão 2015, 37, 313–343. [Google Scholar]
- Zaher, H.; Young, P.S. As coleções zoológicas brasileiras: Panorama e desafios. Cien. Cult. 2003, 55, 24–26. [Google Scholar]
- Carvalho, M.R.; Bockmann, F.A.; Amorim, D.S.; Brandão, C.R.F.; de Vivo, M.; Figueiredo, J.L.; Britski, H.A.; de Pinna, M.C.C.; Menezes, N.A.; Marques, F.P.L.; et al. Taxonomic impediment or impediment to taxonomy? A commentary on systematics and the cybertaxonomic-automation paradigm. Evol. Biol. 2007, 34, 140–143. [Google Scholar] [CrossRef]
- Bockmann, F.A.; Almeida, E.A.B.; Castro, R.M.C.; Garófalo, C.A.; Groppo, M., Jr.; Hsiou, A.S.; Kohlsdorf, T.; Langer, M.C.; Mantelatto, F.L.M. Fund biodiversity collections. Nature 2011, 472, 295. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, S.; Ashby, J. Museums: Campus treasures. Nature 2011, 471, 164–165. [Google Scholar] [CrossRef] [Green Version]
- Matos-Maraví, P.; Wahlberg, N.; Freitas, A.V.L.; Devries, P.; Antonelli, A.; Penz, C.M. Mesoamerica is a cradle and the Atlantic Forest is a museum of Neotropical butterfly diversity: Insights from the evolution and biogeography of Brassolini (Lepidoptera: Nymphalidae). Biol. J. Linn. Soc. 2021. [Google Scholar] [CrossRef]
- Falaschi, R.L.; Amaral, D.T.; Santos, I.; Domingos, A.H.R.; Johnson, G.A.; Martins, A.G.S.; Viroomal, I.B.; Pompéia, S.L.; Mirza, J.D.; Oliveira, A.G.; et al. Neoceroplatus betaryiensis nov. sp. (Diptera: Keroplatidae) is the first record of a bioluminescent fungus-gnat in South America. Sci. Rep. 2019, 9, 11291. [Google Scholar] [CrossRef]
- Costa, C.; Vanin, S.A.; Colepicolo Neto, P. 1986. Larvae of Neotropical Coleoptera, XIV. First record of bioluminescence in the family Staphylinidae (Xantholinini). Rev. Bras. Entomol. 1986, 30, 101–104. [Google Scholar]
- Machado, R.J.P.; Kawada, R.; Rafael, J.A. New continental record and new species of Austromerope (Mecoptera, Meropeidae) from Brazil. ZooKeys 2013, 269, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.V.; Hash, J.M.; Hartop, E.A.; Porras, W.; Amorim, D.S. Baby killers: Documentation and evolution of scuttle fly (Diptera: Phoridae) parasitism of ant (Hymenoptera: Formicidae) brood. Biodivers. Data J. 2017, 5, e11277. [Google Scholar] [CrossRef] [Green Version]
- Viviani, V.R. Fireflies (Coleoptera: Lampyridae) from southeastern Brazil: Habitats, life history, and bioluminescence. Ann. Entomol. Soc. Am. 2001, 94, 129–145. [Google Scholar] [CrossRef]
- Viviani, V.R.; Santos, R.M.D. Bioluminescent Coleoptera of biological station of Boracéia (Salesópolis, SP, Brazil): Diversity, bioluminescence and habitat distribution. Biota Neotrop. 2012, 12, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Silveira, L.F.L.; Mermudes, J.R.M. Memoan ciceroi gen. et sp. nov., a remarkable new firefly genus and species from the Atlantic Rainforest (Coleoptera: Lampyridae). Zootaxa 2013, 3640, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Silveira, L.F.L.; Mermudes, J.R.M. Ybytyramoan, a new genus of fireflies (Coleoptera: Lampyridae, Lampyrinae, Photinini) endemic to the Brazilian Atlantic rainforest, with description of three new species. Zootaxa 2014, 3835, 325–337. [Google Scholar] [CrossRef] [Green Version]
- Silveira, L.F.L.; Souto, P.M.; Mermudes, J.R.M. Four new species of Luciuranus fireflies from the Brazilian Atlantic rainforest (Coleoptera: Lampyridae). Zootaxa 2018, 4413, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Silveira, L.F.L.; Roza, A.S.; Vaz, S.; Mermudes, J.R.M. Description and phylogenetic analysis of a new firefly genus from the Atlantic Rainforest, with five new species and new combinations (Coleoptera: Lampyridae: Lampyrinae). Arthropod Syst. Phylogeny 2021, in press. [Google Scholar]
- Vaz, S.; Silveira, L.F.L.; Rosa, S.P. Morphology and life cycle of a new species of Psilocladus Blanchard, 1846 (Coleoptera, Lampyridae, Psilocladinae), the first known bromeliad-inhabiting firefly. Pap. Avulsos Zool. 2020, 60, e202060(s.i.).24. [Google Scholar] [CrossRef]
- Nascimento, E.A. The current status of knowledge on Lycidae Laporte, 1836 from Brazil (Insecta: Coleoptera). Check List 2013, 9, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Roza, A.S.; Quintino, H.Y.S.; Mermudes, J.R.M.; Silveira, L.F.L. Akamboja gen. nov., a new genus of railroad-worm beetle endemic to the Atlantic Rainforest, with five new species (Coleoptera: Phengodidae, Mastinocerinae). Zootaxa 2017, 4306, 501–523. [Google Scholar] [CrossRef]
- Roza, A.S.; Mermudes, J.R.M. A new genus of railroad-worm beetles from the Atlantic Rainforest from Brazil (Coleoptera: Phengodidae, Mastinocerinae). Pap. Avulsos Zool. 2020, 60, e202060(s.i.).10. [Google Scholar] [CrossRef]
- Ikeda, H.; Nishikawa, M.; Sota, T. Loss of flight promotes beetle diversification. Nat. Comm. 2012, 3, 648. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biffi, G.; Rosa, S.P.; Kundrata, R. Hide-and-Seek with Tiny Neotenic Beetles in One of the Hottest Biodiversity Hotspots: Towards an Understanding of the Real Diversity of Jurasaidae (Coleoptera: Elateroidea) in the Brazilian Atlantic Forest. Biology 2021, 10, 420. https://0-doi-org.brum.beds.ac.uk/10.3390/biology10050420
Biffi G, Rosa SP, Kundrata R. Hide-and-Seek with Tiny Neotenic Beetles in One of the Hottest Biodiversity Hotspots: Towards an Understanding of the Real Diversity of Jurasaidae (Coleoptera: Elateroidea) in the Brazilian Atlantic Forest. Biology. 2021; 10(5):420. https://0-doi-org.brum.beds.ac.uk/10.3390/biology10050420
Chicago/Turabian StyleBiffi, Gabriel, Simone Policena Rosa, and Robin Kundrata. 2021. "Hide-and-Seek with Tiny Neotenic Beetles in One of the Hottest Biodiversity Hotspots: Towards an Understanding of the Real Diversity of Jurasaidae (Coleoptera: Elateroidea) in the Brazilian Atlantic Forest" Biology 10, no. 5: 420. https://0-doi-org.brum.beds.ac.uk/10.3390/biology10050420