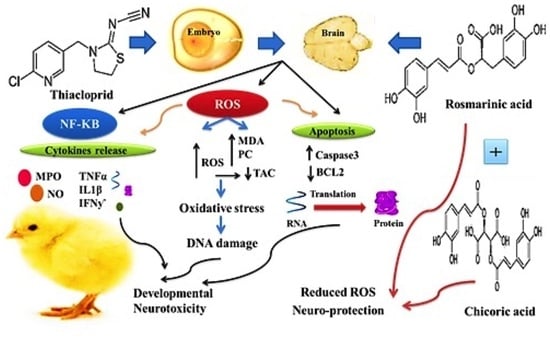
Thiacloprid Induced Developmental Neurotoxicity via ROS-Oxidative Injury and Inflammation in Chicken Embryo: The Possible Attenuating Role of Chicoric and Rosmarinic Acids
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Eggs and Birds
2.3. Experimental Design
2.3.1. Dose-Response
Fertilized Egg and Management
Air Cell Injections
Chicken Embryo Incubation
2.3.2. Antidotal Study
Oxidative Injury Assays in Brain Tissue
Comet Assay
Inflammatory Response Markers
Apoptotic Markers
Transcriptional Analysis of Stress and Inflammatory Cytokine-Related Genes in Brain
2.4. Statistical Analysis
3. Results
3.1. Experiment 1: (Dose-Response)
Effect on Oxidative Stress Biomarkers
3.2. Experiment 2: (Antidotal Study)
3.2.1. Effects on Mortality Rate and Oxidative Stress Variables
3.2.2. Effects on DNA Damage
3.2.3. Effects on Inflammatory Markers
3.2.4. Effects on Apoptotic Markers
3.2.5. Effect on Pro-Inflammatory Cytokines and Stress-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farag, M.R.; Mahmoud, H.K.; El-Sayed, S.A.A.; Ahmed, S.Y.A.; Alagawany, M.; Abou-Zeid, S.M. Neurobehavioral, physiological and inflammatory impairments in response to bifenthrin intoxication in Oreochromis niloticus fish: Role of dietary supplementation with Petroselinum crispum essential oil. Aquat. Toxicol. 2021, 231, 105715. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, S.M.; Aljuaydi, S.H.; AbuBakr, H.O.; Tahoun, E.A.; Di Cerbo, A.; Alagawany, M.; Khalil, S.R.; Farag, M.R. Astaxanthin Mitigates Thiacloprid-Induced Liver Injury and Immunotoxicity in Male Rats. Mar. Drugs 2021, 19, 525. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E.; Durkin, K.A. Neuroactive insecticides: Targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 2013, 58, 99–117. [Google Scholar] [CrossRef]
- Farag, M.R.; Fotoh, M.F.A.-E.; El-Sayed, G.; El-Sayed, E. Modulatory Effect of Ginger Aqueous Extract on Imidacloprid Induced Hepatotoxicity in Rats. Adv. Anim. Vet. Sci. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Farag, M.R.; Abou-EL Fotoh, M.F.; EL-Sayed, G.G.; EL-Sayed, E.W. Modulatory Effect of Ginger Aqueous Extract against Imidacloprid-Induced Neurotoxicity in Rats. Zagazig Vet. J. 2019, 47, 432–446. [Google Scholar] [CrossRef]
- Bonmatin, J.-M.; Noome, D.A.; Moreno, H.; Mitchell, E.A.D.; Glauser, G.; Soumana, O.S.; Bijleveld van Lexmond, M.; Sánchez-Bayo, F. A survey and risk assessment of neonicotinoids in water, soil and sediments of Belize. Environ. Pollut. 2019, 249, 949–958. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the Status and Global Strategy for Neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Casida, J.E.; Quistad, G.B. Golden age of insecticide research: Past, present, or future? Annu. Rev. Entomol. 1998, 43, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Ann, J.; Akk, G. Activation and modulation of human alpha4beta2 nicotinic acetylcholine receptors by the neonicotinoids clothianidin and imidacloprid. J. Neurosci. Res. 2011, 89, 1295–1301. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Taly, A.; Bourreau, J.; De Nardi, F.; Legendre, C.; Henrion, D.; Guerineau, N.C.; Legros, C.; Mattei, C.; Tricoire-Leignel, H. Partial Agonist Activity of Neonicotinoids on Rat Nicotinic Receptors: Consequences over Epinephrine Secretion and In Vivo Blood Pressure. Int. J. Mol. Sci. 2021, 22, 5106. [Google Scholar] [CrossRef]
- Berheim, E.H.; Jenks, J.A.; Lundgren, J.G.; Michel, E.S.; Grove, D.; Jensen, W.F. Effects of Neonicotinoid Insecticides on Physiology and Reproductive Characteristics of Captive Female and Fawn White-tailed Deer. Sci. Rep. 2019, 9, 4534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendawi, M.Y.; Alam, R.T.; Abdellatief, S.A. Ameliorative effect of flaxseed oil against thiacloprid-induced toxicity in rats: Hematological, biochemical, and histopathological study. Environ. Sci. Pollut. Res. Int. 2016, 23, 11855–11863. [Google Scholar] [CrossRef]
- Babelova, J.; Sefcikova, Z.; Cikos, S.; Spirkova, A.; Kovarikova, V.; Koppel, J.; Makarevich, A.V.; Chrenek, P.; Fabian, D. Exposure to neonicotinoid insecticides induces embryotoxicity in mice and rabbits. Toxicology 2017, 392, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Salvaggio, A.; Antoci, F.; Messina, A.; Ferrante, M.; Copat, C.; Ruberto, C.; Scalisi, E.M.; Pecoraro, R.; Brundo, M.V. Teratogenic effects of the neonicotinoid thiacloprid on chick embryos (Gallus gallus domesticus). Food. Chem. Toxicol. 2018, 118, 812–820. [Google Scholar] [CrossRef]
- Kammoun, I.; Sellem, I.; Ben Saad, H.; Boudawara, T.; Nasri, M.; Gharsallah, N.; Mallouli, L.; Amara, I.B. Potential benefits of polysaccharides derived from marine alga Ulva lactuca against hepatotoxicity and nephrotoxicity induced by thiacloprid, an insecticide pollutant. Environ. Toxicol. 2019, 34, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Flanigan, P.M.; Niemeyer, E.D. Effect of cultivar on phenolic levels, anthocyanin composition, and antioxidant properties in purple basil (Ocimum basilicum L.). Food Chem. 2014, 164, 518–526. [Google Scholar] [CrossRef]
- Zhang, H.L.; Dai, L.H.; Wu, Y.H.; Yu, X.P.; Zhang, Y.Y.; Guan, R.F.; Liu, T.; Zhao, J. Evaluation of hepatocyteprotective and anti-hepatitis B virus properties of Cichoric acid from Cichorium intybus leaves in cell culture. Biol. Pharm. Bull. 2014, 37, 1214–1220. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Diao, Z.; Li, J.; Ren, B.; Zhu, D.; Liu, Q.; Liu, Z.; Liu, X. Chicoric acid supplementation ameliorates cognitive impairment induced by oxidative stress via promotion of antioxidant defense system. RSC Adv. 2017, 7, 36149–36162. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Wang, Y.; Du, Q.; Liu, Z.; Liu, X. Cichoric Acid Reverses Insulin Resistance and Suppresses Inflammatory Responses in the Glucosamine-Induced HepG2 Cells. J. Agric. Food Chem. 2015, 63, 10903–10913. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, F.; Zhang, L.; Niu, Y.; Liu, Z.; Liu, X. Comparison of chicoric acid, and its metabolites caffeic acid and caftaric acid: In vitro protection of biological macromolecules and inflammatory responses in BV2 microglial cells. Food Sci. Hum. Wellness 2017, 6, 155–166. [Google Scholar] [CrossRef]
- Makino, T.; Ono, T.; Muso, E.; Honda, G. Inhibitory effect of Perilla frutescens and its phenolic constituents on cultured murine mesangial cell proliferation. Planta Med. 1998, 64, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Simmonds, M.S. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Shimojo, Y.; Kosaka, K.; Noda, Y.; Shimizu, T.; Shirasawa, T. Effect of rosmarinic acid in motor dysfunction and life span in a mouse model of familial amyotrophic lateral sclerosis. J. Neurosci. Res. 2010, 88, 896–904. [Google Scholar] [CrossRef]
- Kim, G.D.; Park, Y.S.; Jin, Y.H.; Park, C.S. Production and applications of rosmarinic acid and structurally related compounds. Appl. Microbiol. Biotechnol. 2015, 99, 2083–2092. [Google Scholar] [CrossRef]
- Fallarini, S.; Miglio, G.; Paoletti, T.; Minassi, A.; Amoruso, A.; Bardelli, C.; Brunelleschi, S.; Lombardi, G. Clovamide and rosmarinic acid induce neuroprotective effects in in vitro models of neuronal death. Br. J. Pharmacol. 2009, 157, 1072–1084. [Google Scholar] [CrossRef] [Green Version]
- Ono, K.; Li, L.; Takamura, Y.; Yoshiike, Y.; Zhu, L.; Han, F.; Mao, X.; Ikeda, T.; Takasaki, J.; Nishijo, H.; et al. Phenolic compounds prevent amyloid beta-protein oligomerization and synaptic dysfunction by site-specific binding. J. Biol. Chem. 2012, 287, 14631–14643. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xu, H.; Jiang, H.; Du, X.; Sun, P.; Xie, J. Neurorescue effect of rosmarinic acid on 6-hydroxydopamine-lesioned nigral dopamine neurons in rat model of Parkinson’s disease. J. Mol. Neurosci. 2012, 47, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Han, J.; Shimozono, H.; Villareal, M.O.; Isoda, H. Caffeoylquinic acid-rich purple sweet potato extract, with or without anthocyanin, imparts neuroprotection and contributes to the improvement of spatial learning and memory of SAMP8 mouse. J. Agric. Food Chem. 2013, 61, 5037–5045. [Google Scholar] [CrossRef]
- Naimi, M.; Vlavcheski, F.; Shamshoum, H.; Tsiani, E. Rosemary Extract as a Potential Anti-Hyperglycemic Agent: Current Evidence and Future Perspectives. Nutrients 2017, 9, 968. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.P.; Wei, H.L.; Zhao, H.S.; Xiao, S.Y.; Zheng, R.L. Antiapoptotic and antioxidant effects of rosmarinic acid in astrocytes. Pharmazie 2005, 60, 62–65. [Google Scholar]
- Hosseinzadeh, H.; Nourbakhsh, M. Effect of Rosmarinus officinalis L. aerial parts extract on morphine withdrawal syndrome in mice. Phytother. Res. 2003, 17, 938–941. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Roncati, L.; Marini, C.; Carnevale, G.; Zavatti, M.; Avallone, R.; Corsi, L. Possible Association Between DHEA and PKCe in Hepatic Encephalopathy Amelioration: A Pilot Study. Front. Vet. Sci. 2021, 8. [Google Scholar] [CrossRef]
- Rashidi, H.; Sottile, V. The chick embryo: Hatching a model for contemporary biomedical research. Bioessays 2009, 31, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Kmecick, M.; Vieira da Costa, M.C.; de Oliveira Ribeiro, C.A.; Ortolani-Machado, C.F. Morphological evidence of neurotoxic effects in chicken embryos after exposure to perfluorooctanoic acid (PFOA) and inorganic cadmium. Toxicology 2019, 427, 152286. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Soloneski, S.; Kujawski, M.; Scuto, A.; Larramendy, M.L. Carbamates: A study on genotoxic, cytotoxic, and apoptotic effects induced in Chinese hamster ovary (CHO-K1) cells. Toxicol. In Vitro 2015, 29, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Franco, R.; Li, S.; Rodriguez-Rocha, H.; Burns, M.; Panayiotidis, M.I. Molecular mechanisms of pesticide-induced neurotoxicity: Relevance to Parkinson’s disease. Chem. Biol. Interact. 2010, 188, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Lushchak, V.I. Contaminant-induced oxidative stress in fish: A mechanistic approach. Fish Physiol. Biochem. 2016, 42, 711–747. [Google Scholar] [CrossRef]
- Kapoor, U.; Srivastava, M.K.; Bhardwaj, S.; Srivastava, L.P. Effect of imidacloprid on antioxidant enzymes and lipid peroxidation in female rats to derive its No Observed Effect Level (NOEL). J. Toxicol. Sci. 2010, 35, 577–581. [Google Scholar] [CrossRef] [Green Version]
- Aydin, B. Effects of thiacloprid, deltamethrin and their combination on oxidative stress in lymphoid organs, polymorphonuclear leukocytes and plasma of rats. Pestic. Biochem. Physiol. 2011, 100, 165–171. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Yang, G.; Weng, H.; Wang, X.; Wang, Q. Changes of enzyme activity and gene expression in embryonic zebrafish co-exposed to beta-cypermethrin and thiacloprid. Environ. Pollut. 2020, 256, 113437. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A. Effect of thiacloprid on early life stages of common carp (Cyprinus carpio). Chemosphere 2018, 194, 481–487. [Google Scholar] [CrossRef]
- Vieira, C.E.D.; Perez, M.R.; Acayaba, R.D.; Raimundo, C.C.M.; Dos Reis Martinez, C.B. DNA damage and oxidative stress induced by imidacloprid exposure in different tissues of the Neotropical fish Prochilodus lineatus. Chemosphere 2018, 195, 125–134. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Abd El-Hack, M.E.; El-Sayed, S.A.A.; Ahmed, S.Y.A.; Samak, D.H. Yucca schidigera extract modulates the lead-induced oxidative damage, nephropathy and altered inflammatory response and glucose homeostasis in Japanese quails. Ecotoxicol. Environ. Saf. 2018, 156, 311–321. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Dawood, M.A.O.; Elnesr, S.S.; Dhama, K. Curcumin and its different forms: A review on fish nutrition. Aquaculture 2021, 532, 736030. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Salah, A.S.; Mahmoud, M.A. The role of oregano herb and its derivatives as immunomodulators in fish. Rev. Aquac. 2020, 12, 2481–2492. [Google Scholar] [CrossRef]
- Alagawany, M.; Nasr, M.; Abdulaziz, A.-A.; Alhotan, R.A.; Azzam, M.M.; Reda, F.M. Impact of dietary cold-pressed chia oil on growth, blood chemistry, haematology, immunity and antioxidant status of growing Japanese quail. Ital. J. Anim. Sci. 2020, 19, 896–904. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.; You, X.; Chen, D.; Li, Y.; Wang, F. Oxidative stress and gene expression of earthworm (Eisenia fetida) to clothianidin. Ecotoxicol. Environ. Saf. 2017, 142, 489–496. [Google Scholar] [CrossRef]
- Schwarzbacherova, V.; Wnuk, M.; Deregowska, A.; Holeckova, B.; Lewinska, A. In vitro exposure to thiacloprid-based insecticide formulation promotes oxidative stress, apoptosis and genetic instability in bovine lymphocytes. Toxicol. In Vitro 2019, 61, 104654. [Google Scholar] [CrossRef]
- Galdikova, M.; Holeckova, B.; Sivikova, K.; Schwarzbacherova, V.; Kolenicova, S. Evaluating the genotoxic damage in bovine whole blood cells in vitro after exposure to thiacloprid. Toxicol. In Vitro 2019, 61, 104616. [Google Scholar] [CrossRef]
- Calderon-Segura, M.E.; Gomez-Arroyo, S.; Villalobos-Pietrini, R.; Martinez-Valenzuela, C.; Carbajal-Lopez, Y.; Calderon-Ezquerro Mdel, C.; Cortes-Eslava, J.; Garcia-Martinez, R.; Flores-Ramirez, D.; Rodriguez-Romero, M.I.; et al. Evaluation of genotoxic and cytotoxic effects in human peripheral blood lymphocytes exposed in vitro to neonicotinoid insecticides news. J. Toxicol. 2012, 2012, 612647. [Google Scholar] [CrossRef] [Green Version]
- Kocaman, A.Y.; Rencuzogullari, E.; Topaktas, M. In vitro investigation of the genotoxic and cytotoxic effects of thiacloprid in cultured human peripheral blood lymphocytes. Environ. Toxicol. 2014, 29, 631–641. [Google Scholar] [CrossRef]
- Çavaş, T.; Çinkılıç, N.; Vatan, Ö.; Yılmaz, D.; Coşkun, M. In vitro genotoxicity evaluation of acetamiprid in CaCo-2 cells using the micronucleus, comet and γH2AX foci assays. Pestic. Biochem. Physiol. 2012, 104, 212–217. [Google Scholar] [CrossRef]
- Bianchi, J.; Cabral-de-Mello, D.C.; Marin-Morales, M.A. Toxicogenetic effects of low concentrations of the pesticides imidacloprid and sulfentrazone individually and in combination in in vitro tests with HepG2 cells and Salmonella typhimurium. Ecotoxicol. Environ. Saf. 2015, 120, 174–183. [Google Scholar] [CrossRef]
- Ge, W.; Yan, S.; Wang, J.; Zhu, L.; Chen, A.; Wang, J. Oxidative stress and DNA damage induced by imidacloprid in zebrafish (Danio rerio). J. Agric. Food Chem. 2015, 63, 1856–1862. [Google Scholar] [CrossRef]
- Ibrahim, K.A.; El-Desouky, M.; Abou-yousef, H.M.; Gabrowny, K.H.; El-Sayed, A. Imidacloprid and/or Esfenvalerate Induce Apoptosis and Disrupt Thyroid Hormones in Neonatal Rats. Glob. J. Biotechnol. Biochem. 2015, 10, 106–112. [Google Scholar] [CrossRef]
- Wu, S.; Li, X.; Liu, X.; Yang, G.; An, X.; Wang, Q.; Wang, Y. Joint toxic effects of triazophos and imidacloprid on zebrafish (Danio rerio). Environ. Pollut. 2018, 235, 470–481. [Google Scholar] [CrossRef]
- Aluigi, M.G.; Guida, C.; Falugi, C. Apoptosis as a specific biomarker of diazinon toxicity in NTera2-D1 cells. Chem. Biol. Interact. 2010, 187, 299–303. [Google Scholar] [CrossRef]
- Khalil, S.R.; Elhakim, Y.A.; Abd El-fattah, A.H.; Ragab Farag, M.; Abd El-Hameed, N.E.; El-Murr, A.E. Dual immunological and oxidative responses in Oreochromis niloticus fish exposed to lambda cyhalothrin and concurrently fed with Thyme powder (Thymus vulgaris L.): Stress and immune encoding gene expression. Fish Shellfish. Immunol. 2020, 100, 208–218. [Google Scholar] [CrossRef]
- DeWitt, D.S.; Prough, D.S. Blast-induced brain injury and posttraumatic hypotension and hypoxemia. J. Neurotrauma. 2009, 26, 877–887. [Google Scholar] [CrossRef]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef]
- Lu, D.Q.; Bei, J.X.; Feng, L.N.; Zhang, Y.; Liu, X.C.; Wang, L.; Chen, J.L.; Lin, H.R. Interleukin-1beta gene in orange-spotted grouper, Epinephelus coioides: Molecular cloning, expression, biological activities and signal transduction. Mol. Immunol. 2008, 45, 857–867. [Google Scholar] [CrossRef]
- Di, H.; He, Q.; Liao, Y.; Kalionis, B.; Tai, X. The Role of Inflammatory Cytokines in the Pathogenesis of Cerebral Palsy. Gynecol. Obstet. 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Arnhold, J.; Osipov, A.N.; Spalteholz, H.; Panasenko, O.M.; Schiller, J. Effects of hypochlorous acid on unsaturated phosphatidylcholines. Free Radic. Biol. Med. 2001, 31, 1111–1119. [Google Scholar] [CrossRef]
- Goyal, S.; Sandhu, H.; Brar, R.S. Histopathological alterations induced after oral sub-acute thiacloprid toxicity in Gallus domesticus. Veterinarski Arhiv 2010, 80, 673–682. [Google Scholar]
- Liu, M.; Chen, L.; Wang, X.; Zhang, W.; Tong, Y.; Ou, L.; Xie, H.; Shen, H.; Ye, X.; Deng, C.; et al. Mercury Export from Mainland China to Adjacent Seas and Its Influence on the Marine Mercury Balance. Environ. Sci. Technol. 2016, 50, 6224–6232. [Google Scholar] [CrossRef]
- Ding, X.; Jian, T.; Li, J.; Lv, H.; Tong, B.; Li, J.; Meng, X.; Ren, B.; Chen, J.L. Chicoric Acid Ameliorates Nonalcoholic Fatty Liver Disease via the AMPK/Nrf2/NFκB Signaling Pathway and Restores Gut Microbiota in High-Fat-Diet-Fed Mice. Oxidative Med. Cell. Longev. 2020, 2020, 9734560. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, H.S.; Park, E.; Kim, S.; Lee, S.Y.; Kim, C.S.; Kim, D.K.; Kim, S.J.; Chun, H.S. Rosmarinic acid protects human dopaminergic neuronal cells against hydrogen peroxide-induced apoptosis. Toxicology 2008, 250, 109–115. [Google Scholar] [CrossRef]
- Ghasemzadeh Rahbardar, M.; Amin, B.; Mehri, S.; Mirnajafi-Zadeh, S.J.; Hosseinzadeh, H. Anti-inflammatory effects of ethanolic extract of Rosmarinus officinalis L. and rosmarinic acid in a rat model of neuropathic pain. Biomed. Pharmacother. 2017, 86, 441–449. [Google Scholar] [CrossRef]
- Coelho, V.R.; Vieira, C.G.; de Souza, L.P.; Moyses, F.; Basso, C.; Papke, D.K.; Pires, T.R.; Siqueira, I.R.; Picada, J.N.; Pereira, P. Antiepileptogenic, antioxidant and genotoxic evaluation of rosmarinic acid and its metabolite caffeic acid in mice. Life Sci. 2015, 122, 65–71. [Google Scholar] [CrossRef]
- Lv, R.; Du, L.; Liu, X.; Zhou, F.; Zhang, Z.; Zhang, L. Rosmarinic acid attenuates inflammatory responses through inhibiting HMGB1/TLR4/NF-kappaB signaling pathway in a mouse model of Parkinson’s disease. Life Sci. 2019, 223, 158–165. [Google Scholar] [CrossRef]
- Ramalho, L.N.; Pasta, A.A.; Terra, V.A.; Augusto, M.; Sanches, S.C.; Souza-Neto, F.P.; Cecchini, R.; Gulin, F.; Ramalho, F.S. Rosmarinic acid attenuates hepatic ischemia and reperfusion injury in rats. Food. Chem. Toxicol. 2014, 74, 270–278. [Google Scholar] [CrossRef]
- Raskovic, A.; Milanovic, I.; Pavlovic, N.; Cebovic, T.; Vukmirovic, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef] [Green Version]
- Qiao, S.; Li, W.; Tsubouchi, R.; Haneda, M.; Murakami, K.; Takeuchi, F.; Nisimoto, Y.; Yoshino, M. Rosmarinic acid inhibits the formation of reactive oxygen and nitrogen species in RAW264.7 macrophages. Free Radic. Res. 2005, 39, 995–1003. [Google Scholar] [CrossRef]






| Gene | Sequences (5′-3′) | Accession Number |
|---|---|---|
| Caspase 3, apoptosis-related cysteine peptidase (Casp-3) | F: TGTGGACTCTGGAATTCTGCC R: AACGAGATGACAGTCCGGTA | NM_204725 |
| B-cell CLL/lymphoma 2 (Bcl-2) | F: ATCGTCGCCTTCTTCGAGTT R: AGGCATCCCATCCTCCGTT | NM_205339 |
| Interferon, gamma (IFN-γ) | F: GAACTGGACAGAGAGAAATGAGA R: ATGTGTTTGATGTGCGGCTT | NM_205149 |
| Tumor necrosis factor-alpha (TNF-α) | F: TGCTGTTCTATGACCGCC R: CTTTCAGAGCATCAACGCA | AY765397 |
| Interleukin-1beta (IL-1β) | F: CTACACCCGCTCACAGTCCT R: GCCTCACTTTCTGGCTGGA | NM_204524 |
| Nuclear factor kappa (NF-κB1) | F: TACCGGGAACAACACCACTG R: CAGAGGGCCTTGTGACAGTA | NM_205134 |
| Beta actin (β-actin) | F: CCCAAAGCCAACAGAGAGAA R: CCATCACCAGAGTCCATCAC | NM_205518 |
| Parameters | Experimental Groups | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | TH | CA | RA | TH/CA | TH/RA | TH/CA + RA | ||
| MDA (nmol/mg protein) | 16.29 ± 0.07 bcd | 21.81 ± 1.83 a | 14.18 ± 0.66 cd | 13.00 ± 0.57 d | 18.20 ± 0.14 b | 17.84 ± 0.20 b | 17.35 ± 0.62 bc | <0.001 |
| PC (nmol/mg protein) | 6.05 ± 0.03 c | 12.44 ± 0.12 a | 5.14 ± 0.06 d | 5.00 ± 0.00 d | 8.23 ± 0.16 b | 7.73 ± 0.25 b | 6.71 ± 0.29 c | <0.001 |
| TAC (mM/g protein) | 3.53 ± 0.01 ab | 1.46 ± 0.02 e | 3.61 ± 0.01 ab | 3.81 ± 0.08 a | 2.11 ± 0.00 d | 2.67 ± 0.14 c | 3.34 ± 0.12 b | <0.001 |
| ROS (U/mg protein) | 12.56 ± 0.03 cde | 23.36 ± 2.88 a | 9.28 ± 0.53 de | 7.74 ± 0.23 e | 19.20 ± 0.47 ab | 17.38 ± 0.62 bc | 13.99 ± 0.38 cd | <0.001 |
| Parameters | Experimental Groups | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | TH | CA | RA | TH/CA | TH/RA | TH/CA + RA | ||
| Inflammatory Markers | ||||||||
| TNF-α (pg/mg protein) | 59.80 ± 0.77 d | 93.58 ± 2.32 a | 64.36 ± 1.492 d | 47.58 ± 4.32 e | 80.23 ± 4.02 b | 72.15 ± 3.09 cd | 62.36 ± 1.72 d | <0.001 |
| IL-1β (pg/mg protein) | 81.16 ± 0.55 d | 150.16 ± 1.15 a | 73.91 ± 2.71 e | 64.67 ± 1.09 f | 128.42 ± 3.17 b | 100.18 ± 0.04 c | 87.59 ± 1.45 d | <0.001 |
| NO (µmol/g protein) | 25.17 ± 0.10 de | 51.51 ± 0.89 a | 21.95 ± 0.32 ef | 20.27 ± 0.19 f | 37.78 ± 1.58 b | 32.03 ± 0.69 c | 29.21 ± 01.97 cd | <0.001 |
| MPO (ng/mg protein) | 1.07 ± 0.01 d | 12.74 ± 0.70 a | 1.20 ± 0.19 d | 1.15 ± 0.08 d | 7.24 ± 0.06 b | 3.81 ± 0.30 c | 1.37 ± 0.06 d | <0.001 |
| Apoptotic biomarkers | ||||||||
| Casp3 (ng/mg protein) | 1.51 ± 0.00 d | 6.15 ± 0.02 a | 1.62 ± 0.11 d | 1.72 ± 0.07 d | 4.05 ± 0.33 b | 3.56 ± 0.02 b | 2.64 ± 0.20 c | <0.001 |
| Bcl-2 (ng/mg protein) | 0.41 ± 0.00 b | 0.15 ± 00.00 d | 0.53 ± 0.02 a | 0.61 ± 0.03 a | 0.26 ± 0.03 cd | 0.30 ± 0.05 bc | 0.38 ± 0.01 bc | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farag, M.R.; Khalil, S.R.; Zaglool, A.W.; Hendam, B.M.; Moustafa, A.A.; Cocco, R.; Di Cerbo, A.; Alagawany, M. Thiacloprid Induced Developmental Neurotoxicity via ROS-Oxidative Injury and Inflammation in Chicken Embryo: The Possible Attenuating Role of Chicoric and Rosmarinic Acids. Biology 2021, 10, 1100. https://0-doi-org.brum.beds.ac.uk/10.3390/biology10111100
Farag MR, Khalil SR, Zaglool AW, Hendam BM, Moustafa AA, Cocco R, Di Cerbo A, Alagawany M. Thiacloprid Induced Developmental Neurotoxicity via ROS-Oxidative Injury and Inflammation in Chicken Embryo: The Possible Attenuating Role of Chicoric and Rosmarinic Acids. Biology. 2021; 10(11):1100. https://0-doi-org.brum.beds.ac.uk/10.3390/biology10111100
Chicago/Turabian StyleFarag, Mayada R., Samah R. Khalil, Asmaa W. Zaglool, Basma M. Hendam, Amr A. Moustafa, Raffaella Cocco, Alessandro Di Cerbo, and Mahmoud Alagawany. 2021. "Thiacloprid Induced Developmental Neurotoxicity via ROS-Oxidative Injury and Inflammation in Chicken Embryo: The Possible Attenuating Role of Chicoric and Rosmarinic Acids" Biology 10, no. 11: 1100. https://0-doi-org.brum.beds.ac.uk/10.3390/biology10111100