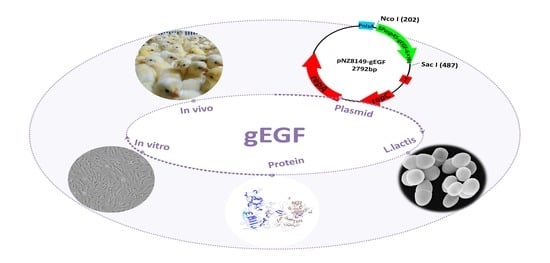
Expression of Gallus Epidermal Growth Factor (gEGF) with Food-Grade Lactococcus lactis Expression System and Its Biological Effects on Broiler Chickens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Medium for Bacterial Growth
2.2. Construction of the Recombinant Plasmid pNZ8149-gEGF and Transformation of Lactococcus Lactis
2.3. Expression of Recombinant gEGF Protein in Lactococcus Lactis
2.4. Purification of Recombinant gEGF Protein from Lactococcus Lactis
2.5. In Vitro Experiment
2.6. In Vivo Animal Experiment
2.7. Statistical Analysis
3. Results
3.1. Expression of gEGF Protein in a Bacterial Culture Supernatant
3.2. gEGF Promotes UMNSAH/DF-1 Cell Proliferation
3.3. gEGF Promotes the Growth Performance of Broilers
3.4. gEGF Improves the Immune Function of Broilers
3.5. gEGF Promotes the Intestinal Development of Broilers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
References
- Fagbemi, A.O.; Wright, N.; Lakhoo, K.; Edwards, A.D. Immunoreactive Epidermal Growth Factor Receptors Are Present in Gastrointestinal Epithelial Cells of Preterm Infants with Necrotising Enterocolitis. Early Hum. Dev. 2001, 65, 1–9. [Google Scholar] [CrossRef]
- Clark, J.A.; Lane, R.H.; Maclennan, N.K.; Holubec, H.; Dvorakova, K.; Halpern, M.D.; Williams, C.S.; Payne, C.M.; Dvorak, B. Epidermal Growth Factor Reduces Intestinal Apoptosis in an Experimental Model of Necrotizing Enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G755–G762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakai, K.; Hamada, Y.; Kato, Y.; Kitagawa, K.; Hioki, K.; Ito, S.; Okumura, T. Further Evidence That Epidermal Growth Factor Enhances the Intestinal Adaptation Following Small Bowel Transplantation. Life Sci. 2004, 75, 2091–2102. [Google Scholar] [CrossRef]
- Cohen, S. Isolation of a Mouse Submaxillary Gland Protein Accelerating Incisor Eruption and Eyelid Opening in the New-Born Animal. J. Biol. Chem. 1962, 237, 1555–1562. [Google Scholar] [CrossRef]
- Klingensmith, N.J.; Yoseph, B.P.; Liang, Z.; Lyons, J.D.; Burd, E.M.; Margoles, L.M.; Koval, M.; Ford, M.L.; Coopersmith, C.M. Epidermal Growth Factor Improves Intestinal Integrity and Survival in Murine Sepsis Following Chronic Alcohol Ingestion. Shock 2017, 47, 184–192. [Google Scholar] [CrossRef]
- Grupcev, G.; Wallin, C.; Emås, S.; Theodorsson, E.; Hellström, P.M. Transforming Growth Factor-Alpha and Epidermal Growth Factor Inhibit Gastric Acid Secretion and Stimulate Release of Somatostatin and Neurotensin in the Conscious Rat. Regul. Pept. 1994, 52, 111–118. [Google Scholar] [CrossRef]
- Schultz, G.A.; Heyner, S. Growth Factors in Preimplantation Mammalian Embryos. Oxford Rev. Reprod. B 1993, 15, 43. [Google Scholar]
- Dadi, T.D.; Li, M.W.; Lloyd, K.C. EGF and TGF-Alpha Supplementation Enhances Development of Cloned Mouse Embryos. Cloning Stem Cells 2007, 9, 315–326. [Google Scholar] [CrossRef]
- Ledeganck, K.J.; Boulet, G.A.; Bogers, J.J.; Verpooten, G.A.; De, W.B.Y.; Chulso, M. The TRPM6/EGF Pathway Is Downregulated in a Rat Model of Cisplatin Nephrotoxicity. PLoS ONE 2013, 8, e57016. [Google Scholar] [CrossRef] [Green Version]
- Pavlov, T.S.; Levchenko, V.; O’Connor, P.M.; Ilatovskaya, D.V.; Palygin, O.; Mori, T.; Mattson, D.L.; Sorokin, A.; Lombard, J.H.; Cowley, A.W., Jr.; et al. Deficiency of Renal Cortical EGF Increases ENaC Activity and Contributes to Salt-Sensitive Hypertension. J. Am. Soc. Nephrol. 2013, 24, 1053–1062. [Google Scholar] [CrossRef] [Green Version]
- Bracher, A.; Cardona, A.S.; Tauber, S.; Fink, A.M.; Steiner, A.; Pehamberger, H.; Niederleithner, H.; Petzelbauer, P.; Gröger, M.; Loewe, R. Epidermal Growth Factor Facilitates Melanoma Lymph Node Metastasis by Influencing Tumor Lymphangiogenesis. J. Invest. Dermatol. 2013, 133, 230–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.H.; Chiang, K.H.; Shieh, J.M.; Huang, C.R.; Shen, C.J.; Huang, W.C.; Chen, B.K. Epidermal Growth Factor-Induced ANGPTL4 Enhances Anoikis Resistance and Tumour Metastasis in Head and Neck Squamous Cell Carcinoma. Oncogene 2017, 36, 2228–2242. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Sakamoto, S.; Miyoshi, K.; Fuwa, T.; Yoda, K.; Yamasaki, M.; Tamura, G.; Miyake, T. Synthesis and Secretion of Human Epidermal Growth Factor by Escherichia coli. Proc. Natl. Acad. Sci. USA 1985, 82, 7212–7216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.G.; Min, M.K.; Ahn, S.C. Expression of a Fusion Protein Containing Human Epidermal Growth Factor and the Collagen-Binding Domain of Vibrio. Biotechnol. Lett. 2009, 31, 259–264. [Google Scholar] [CrossRef]
- Yamagata, H.; Nakahama, K.; Suzuki, Y. Use of Bacillus brevis for Efficient Synthesis and Secretion of Human Epidermal Growth Factor. Proc. Natl. Acad. Sci. USA 1989, 86, 3589–3593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebisu, S.; Takagi, H.; Kadowaki, K.; Yamagata, H.; Udaka, S. The Efficient Production of Human Epidermal Growth Factor by Bacillus brevis. Ann. N. Y. Acad. Sci. 1996, 782, 115–122. [Google Scholar] [CrossRef]
- Urdea, M.S.; Merryweather, J.P.; Mullenbach, G.T.; Coit, D.; Heberlein, U.; Valenzuela, P.; Barr, P.J. Chemical Synthesis of a Gene for Human Epidermal Growth Factor Urogastrone and Its Expression in Yeast. Proc. Natl. Acad. Sci. USA 1983, 80, 7461–7465. [Google Scholar] [CrossRef] [Green Version]
- Brake, A.J.; Merryweather, J.P.; Coit, D.G.; Heberlein, U.A.; Masiarz, F.R.; Mullenbach, G.T.; Urdea, M.S.; Valenzuela, P.; Barr, P.J. α-Factor-Directed Synthesis and Secretion of Mature Foreign Proteins in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1984, 81, 4642–4646. [Google Scholar] [CrossRef] [Green Version]
- Bedford, A.; Huynh, E.; Fu, M.; Zhu, C.; Wey, D.; De, L.C.; Li, J.L. Growth Performance of Early-Weaned Pigs Is Enhanced by Feeding Epidermal Growth Factor-Expressing Lactococcus lactis Fermentation Product. J. Biotechnol. 2014, 173, 47–52. [Google Scholar] [CrossRef]
- Lee, D.N.; Kuo, T.Y.; Chen, M.C.; Tang, T.Y.; Liu, F.H.; Weng, C.F. Expression of Porcine Epidermal Growth Factor in Pichia pastoris and Its Biology Activity in Early-Weaned Piglets. Life Sci. 2006, 78, 649–654. [Google Scholar] [CrossRef]
- Elliott, S.N.; Wallace, J.L.; Mcknight, W.; Gall, D.G.; Hardin, J.A.; Olson, M.; Buret, A. Bacterial Colonization and Healing of Gastric Ulcers: The Effects of Epidermal Growth Factor. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, G105–G112. [Google Scholar] [CrossRef] [Green Version]
- Lambrosteski, J.M.; Kalischuk, L.D.; Inglis, G.D.; Buret, A.G. Epidermal Growth Factor Inhibits Campylobacter jejuni-Induced Claudin-4 Disruption, Loss of Epithelial Barrier Function, and Escherichia coli. Translocation. Infect. Immun. 2008, 76, 3390–3398. [Google Scholar] [CrossRef] [Green Version]
- Donovan, S.M.; Zijlstra, R.T.; Odle, J. Use of the Piglet to Study the Role of Growth Factors in Neonatal Intestinal Development. Endocr. Regul. 1994, 28, 153–162. [Google Scholar] [PubMed]
- Kim, E.; Leung, H.; Akhtar, N.; Li, J.; Barta, J.R.; Wang, Y.; Yang, C.; Kiarie, E. Growth Performance and Gastrointestinal Responses of Broiler Chickens Fed Corn-Soybean Meal Diet without or with Exogenous Epidermal Growth Factor upon Challenge with Eimeria. Poult. Sci. 2017, 96, 3676–3686. [Google Scholar] [CrossRef] [PubMed]
- Yasui, S.; Naga, A.; Oohira, A.; Iwashita, M.; Konno, K. Effects of Anti-Mouse EGF Antiserum on Prenatal Lung Development in Fetal Mice. Pediatr. Pulmonol. 1993, 15, 251–256. [Google Scholar] [CrossRef]
- Carpenter, G.; Cohen, S. Human Epidermal Growth Factor and the Proliferation of Human Fibroblasts. J. Cell. Physiol. 1976, 88, 227–237. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, P.M.; Connerton, I.F.; White, K.L. Pilot Study of Long-Term Anaesthesia in Broiler Chickens. Vet. Anaesth. Analg. 2016, 43, 72–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeger, L.A.; Lamar, C.H.; Cline, T.R.; Cardona, C.J. Effect of Orally Administered Epidermal Growth Factor on the Jejunal Mucosa of Weaned Pigs. Am. J. Vet. Res. 1990, 51, 471–474. [Google Scholar]
- Wang, B.; Yang, X.; Wu, R. High-Level Production of the Mouse Epidermal Growth Factor in a Bacillus brevis Expression System. Protein Expr. Purif. 1993, 4, 223–231. [Google Scholar] [CrossRef]
- Shimizu, N.; Fukuzono, S.; Harada, Y.; Fujimori, K.; Gotoh, K.; Yamazaki, Y. Mass Production of Human Epidermal Growth Factor Using Fed-Batch Cultures of Recombinant Escherichia coli. Biotechnol. Bioeng. 2010, 38, 37–42. [Google Scholar] [CrossRef]
- Mierau, I.; Olieman, K.; Mond, J.; Smid, E.J. Optimization of the Lactococcus lactis nisin-controlled gene expression system NICE for industrial applications. Microb. Cell Fact. 2005, 4, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brink, B.T.; Otto, R.; Hansen, U.P.; Konings, W.N. Energy Recycling by Lactate Efflux In growing and Nongrowing Cells of Streptococcus cremoris. J. Bacteriol. 1985, 162, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, R.T. Genomic Analysis of High-Cell-Density Recombinant Escherichia coli Fermentation and “Cell Conditioning” for Improved Recombinant Protein Yield. Biotechnol. Bioeng. 2015, 72, 85–95. [Google Scholar] [CrossRef]
- Ozturk, A.M.; Sozbilen, M.C.; Sevgili, E.; Dagci, T.; Özyalcin, H.; Armagan, G. Epidermal Growth Factor Regulates Apoptosis and Oxidative Stress in a Rat Model of Spinal Cord Injury. Injury 2018, 49, 1038–1045. [Google Scholar] [CrossRef]
- Aoyagi, T.; Kato, N.; Suya, H.; Miura, Y.; Yoshida, T.; Ohura, T. Effects of Mouse and Human Epidermal Growth Factor on the Outgrowing Epidermis of Pig and Human Skin Explants. J. Dermatol. 1984, 11, 519–522. [Google Scholar] [CrossRef]
- Lax, I.; Johnson, A.; Howk, R.; Sap, J.; Givol, D. Chicken Epidermal Growth Factor (EGF) Receptor: cDNA Cloning, Expression in Mouse Cells, and Differential Binding of EGF and Transforming Growth Factor Alpha. Mol. Cell. Biol. 1988, 8, 1970–1978. [Google Scholar] [CrossRef] [Green Version]
- Yu, A.; Matsuda, Y.; Takeda, A.; Uchinuma, E.; Kuroyanagi, Y. Effect of EGF and bFGF on Fibroblast Proliferation and Angiogenic Cytokine Production from Cultured Dermal Substitutes. J. Biomater. Sci. Polym. Ed. 2012, 23, 1315–1324. [Google Scholar] [CrossRef]
- Wang, D.; Xu, S.; Lin, Y.; Fang, Z.; Che, L.; Xue, B.; Wu, D. Recombinant Porcine Epidermal Growth Factor-Secreting Lactococcus lactis Promotes the Growth Performance of Early-Weaned Piglets. BMC Vet. Res. 2014, 10, 171. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, G.; Cohen, S. Epidermal Growth Factor. J. Biol. Chem. 1990, 265, 7709–7712. [Google Scholar] [CrossRef]
- Bi, Q.; Zhang, Q.; Ma, J.; Xu, M.; Zhang, S.J.; Qiu, B.S.; Xia, B.; Gu, H.F.; Hong, J.F.; Zhao, C.; et al. Effect of Combination Therapy with Alginate Dressing and Mouse Epidermal Growth Factor on Epidermal Stem Cells in Patients with Refractory Wounds. Chin. Med. J. 2012, 125, 257–261. [Google Scholar]
- Miettinen, P.J.; Perheentupa, J.; Otonkoski, T.; Lähteenmäki, A.; Panula, P. EGF- and TGF-Alpha-Like Peptides in Human Fetal Gut. Pediatr. Res. 1989, 26, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odle, J.; Zijlstra, R.T.; Donovan, S.M. Intestinal Effects of Milkborne Growth Factors in Neonates of Agricultural Importance. J. Anim. Sci. 1996, 74, 2509–2522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, P.; Toms, D.; Yin, Y.L.; Cheung, Q.; Gong, J.H.; Lange, K.D.; Li, J.L. Epidermal Growth Factor-Expressing Lactococcus lactis Enhances Intestinal Development of Early-Weaned Pigs. J. Nutr. 2010, 140, 806–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, W.H.; Xu, R.J. Stability of Epidermal Growth Factor in the Gastrointestinal Lumen of Suckling and Weaned Pigs. Life Sci. 1996, 59, 197–208. [Google Scholar] [CrossRef]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Brenes, A. Effects of Dietary Polyphenol-Rich Grape Products on Intestinal Microflora and Gut Morphology in Broiler Chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef]
- Thompson, J.S. Epidermal Growth Factor Inhibits Somatostatin-Induced Apoptosis. J. Surg. Res. 1999, 81, 95–100. [Google Scholar] [CrossRef]






| Items | Content (%) |
|---|---|
| Ingredients (%) | |
| Corn, yellow | 58.10 |
| Soybean meal, dehulled (CP, 48%) | 30 |
| Fish meal | 4.94 |
| Soybean oil | 3.0 |
| Calcium bicarbonate | 1.4 |
| Limestone | 1.4 |
| Salt | 0.25 |
| L-Lysine-HCl | 0.03 |
| DL-methionine | 0.2 |
| Choline chloride | 0.13 |
| Antioxidant | 0.05 |
| Mineral and vitamin premix (supplied per kilogram) | 0.05 |
| Nutrient content (%) | |
| Crude protein (%) | 21 |
| Crude fiber (%) | 4.95 |
| Calcium (%) | 0.95 |
| Phosphorus (%) | 0.62 |
| Lysine (%) | 1.30 |
| Methionine (%) | 0.55 |
| Met + Cys (%) | 0.90 |
| ME (kcal/kg) | 3100 |
| Item | Time | Treatment | SEM | p-Value | ||
|---|---|---|---|---|---|---|
| Control | P-LL | gEGF | ||||
| IABW (g) | 91.43 | 92.00 | 91.42 | 1.79 | 0.930 | |
| ABW (g) | Week 1 | 210.47 a | 225.52 a | 238.62 b | 5.62 | 0.018 |
| Week 2 | 395.14 a | 390.19 a | 442.10 b | 12.46 | 0.006 | |
| ADFI (g) | Week 1 | 30.43 a | 31.12 a | 33.57 b | 0.71 | 0.011 |
| Week 2 | 33.12 a | 32.65 a | 36.40 b | 1.02 | 0.020 | |
| ADG (g/d) | Week 1 | 17.01 a | 19.10 a | 21.03 b | 0.67 | 0.007 |
| Week 2 | 26.59 a | 26.08 a | 29.87 b | 1.32 | 0.018 | |
| Gain:feed (g/g) | Week 1 | 0.56 a | 0.61 b | 0.63 b | 0.17 | 0.016 |
| Week 2 | 0.80 a | 0.80 a | 0.82 b | 0.06 | 0.027 | |
| Item | Treatment | SEM | p-Value | ||
|---|---|---|---|---|---|
| Control | P-LL | gEGF | |||
| BW (g) | 397.11 a | 395.34 a | 446.67 b | 4.29 | <0.001 |
| Spleen weight (g) | 0.38 a | 0.38 a | 0.57 b | 0.28 | 0.001 |
| Index of spleen (spleen weight (mg)/BW (g)) | 0.95 a | 0.97 a | 1.28 b | 0.06 | 0.002 |
| BF weight (g) | 1.43 a | 1.31 a | 1.69 b | 0.62 | 0.002 |
| Index of BF (BF weight (mg)/BW (g)) | 3.59 a,b | 3.30 a | 3.78 b | 0.14 | 0.040 |
| TG weight (g) | 2.18 a | 2.24 a | 2.86 b | 0.77 | <0.001 |
| Index of TG(TG weight (mg)/BW (g)) | 5.50 a | 5.66 a | 6.40 b | 0.20 | 0.008 |
| Item | Treatment | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| Control | P-LL | gEGF | ||||
| Serum | IgA (mg/mL) | 0.27 a | 0.23 a | 0.30 b | 0.01 | 0.004 |
| IgG (mg/mL) | 4.07 a | 3.96 a | 5.09 b | 0.34 | 0.010 | |
| Mucosal sIgA (ng/mg) | Duodenum | 197.20 a | 193.80 a | 371.34 b | 8.13 | <0.001 |
| Jejunum | 226.65 | 247.90 | 219.22 | 26.27 | 0.559 | |
| Ileum | 619.85 | 603.26 | 640.77 | 26.82 | 0.428 | |
| Item | Treatment | SEM | p-Value | ||
|---|---|---|---|---|---|
| Control | P-LL | gEGF | |||
| Duodenum | |||||
| Villus height (μm) | 1116.11 a | 1210.01 a | 1333.72 b | 41.85 | 0.006 |
| Crypt depth (μm) | 97.17 a,b | 97.08 a | 84.79 b | 6.32 | 0.016 |
| VH/CD | 11.59 a | 12.47 a | 15.73 b | 0.64 | 0.001 |
| Jejunum | |||||
| Villus height (μm) | 763.91 a | 757.41 a | 951.30 b | 16.90 | <0.001 |
| Crypt depth (μm) | 97.63 a | 100.03 a | 130.51 b | 7.31 | 0.007 |
| VH/CD | 7.87 | 7.62 | 7.29 | 0.47 | 0.504 |
| Ileum | |||||
| Villus height (μm) | 652.11 a | 648.67 a | 695.15 b | 13.72 | 0.026 |
| Crypt depth (μm) | 82.94 | 85.01 | 88.47 | 6.07 | 0.673 |
| VH/CD | 7.86 | 7.72 | 7.87 | 0.50 | 0.949 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Chen, P.; Shi, S.; Li, X.; Shi, D.; Zhou, Z.; Li, Z.; Xiao, Y. Expression of Gallus Epidermal Growth Factor (gEGF) with Food-Grade Lactococcus lactis Expression System and Its Biological Effects on Broiler Chickens. Biomolecules 2021, 11, 103. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11010103
Zhou Y, Chen P, Shi S, Li X, Shi D, Zhou Z, Li Z, Xiao Y. Expression of Gallus Epidermal Growth Factor (gEGF) with Food-Grade Lactococcus lactis Expression System and Its Biological Effects on Broiler Chickens. Biomolecules. 2021; 11(1):103. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11010103
Chicago/Turabian StyleZhou, Yu, Pinpin Chen, Shuai Shi, Xiaowen Li, Deshi Shi, Zutao Zhou, Zili Li, and Yuncai Xiao. 2021. "Expression of Gallus Epidermal Growth Factor (gEGF) with Food-Grade Lactococcus lactis Expression System and Its Biological Effects on Broiler Chickens" Biomolecules 11, no. 1: 103. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11010103