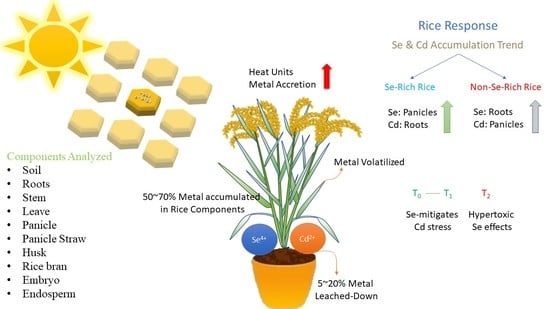
Cross-Talk between Cadmium and Selenium at Elevated Cadmium Stress Determines the Fate of Selenium Uptake in Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Planting Conditions
2.3. Nutrient Treatments Used in the Experiment
2.4. Selenium and Cadmium Stress Treatment Groups
2.5. Sample Preparation and Metal Analysis
2.5.1. Determination of Total Se in Samples
2.5.2. RGF-6800 Parameter Settings
2.5.3. Determination of Cd Contents in Samples
2.5.4. iCETM-3300 Machine Parameters and Lamp Settings
2.6. Preparation of Soil Powder
Determination of Se and Cd in Soil Powder
2.7. Estimation of Heat Units for Each Respective Stage
2.8. Statistical Analysis
3. Results and Discussion
3.1. Cd and Se Activity in the Soil
3.2. Elemental Uptake and Recovery Rate
3.3. Vegetative versus Reproductive Rice Tissues, Metal’s Uptake Trend
3.4. Health Risks and Milling Losses in Cd and Se-Treated Rice
3.5. Effect of Heat Units on Rice Nutritional Status
4. Conclusions
- The increased fertilizer treatment in soil bulk linearly increased the metal contents in rice grain.
- Rice tissues uptake 50–70% of metal applied, while 5–20% leached down into the soil.
- The Cd concentration in polished rice of Se-rich rice was below the threshold level of Cd (0.2 mg kg−1), when Se was applied at a rate of 0.4 mg kg−1 at tillering stage.
- Panicles and rice bran accumulated a maximum of metal contents. At low Cd stress (1 mg kg−1), the metal accumulation in rice tissues was within a safe range, while at high Cd stress (2 mg kg−1), a hyper accumulative Se effect was observed in polished rice.
- The late matured rice cultivars consumed more heat units, and more metal contents were assessed in them.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdisicpl Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamers, A.; Blust, R.; De Coen, W.; Griffin, J.L.; Jones, O.A. An omics based assessment of cadmium toxicity in the green alga Chlamydomonas reinhardtii. Aqua Toxicol. 2013, 126, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Gratão, P.L.; Polle, A.; Lea, P.J.; Azevedo, R.A. Making the life of heavy metal-stressed plants a little easier. Funt. Plant. Biol. 2005, 32, 481–494. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, Y.; Zhu, Y.G.; Tang, Z.; Mcgrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2015, 49, 750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, W. Advances on physiological andecological effects of cadmiumonplants. Acta Ecol. Sin. 2000, 20, 514–523. [Google Scholar]
- Aitio, A.; Tritscher, A. Effects on health of cadmium-WHO approaches and conclusions. Biometals 2004, 17, 491. [Google Scholar] [CrossRef]
- Kjellström, T. Exposure and accumulation of cadmium in populations from Japan, the United States, and Sweden. Environ. Health Persp. 1979, 28, 169–197. [Google Scholar] [CrossRef] [PubMed]
- Pachuau, L.; Dutta, R.S.; Roy, P.K.; Kalita, P.; Lalhlenmawia, H. Physicochemical and disintegrant properties of glutinous rice starch of Mizoram, India. Int. J. Biol. Macromol. 2017, 95, 1298–1304. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.; Wahid, A.; Khaliq, A.; Kobayashi, N. Rice seed invigoration: A review. In Organic Farming, Pest Control and Remediation of Soil Pollutants; Springer: Berlin/Heidelberg, Germany, 2009; pp. 137–175. [Google Scholar]
- Rehman, M.Z.; Rizwan, M.; Rauf, A.; Ayub, M.A.; Ali, S.; Qayyum, M.F.; Waris, A.A.; Naeem, A.; Sanaullah, M. Split application of silicon in cadmium (Cd) spiked alkaline soil plays a vital role in decreasing Cd accumulation in rice (Oryza sativa L.) grains. Chemosphere 2019, 226, 454–462. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, X.; Zhu, J.; Sapkota, A.; Meng, B.; Yao, H.; Qin, H.; Larssen, T. Selenium in soil inhibits mercury uptake and translocation in rice (Oryza sativa L.). Environ. Sci. Technol. 2012, 46, 10040–10046. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, W.; Dai, H.; Cao, F.; Zhang, G.; Wu, F. Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J. Hazard. Mater. 2012, 235, 343–351. [Google Scholar] [CrossRef]
- Zwolak, I.; Zaporowska, H. Selenium interactions and toxicity: A review. Selenium interactions and toxicity. Cell Biol. Toxicol. 2012, 28, 31–46. [Google Scholar] [CrossRef]
- Nawaz, F.; Ahmad, R.; Ashraf, M.Y.; Waraich, E.A.; Khan, S.Z. Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotoxicol. Environ. Saf. 2015, 113, 191–200. [Google Scholar] [CrossRef]
- El Mehdawi, A.F.; Jiang, Y.; Guignardi, Z.S.; Esmat, A.; Pilon, M.; Pilon-Smits, E.A.H.; Schiavon, M. Influence of sulfate supply on selenium uptake dynamics and expression of sulfate/selenate transporters in selenium hyperaccumulator and nonhyperaccumulator Brassicaceae. New Phytol. 2018, 217, 194–205. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A. The fascinating facets of plant selenium accumulation-biochemistry, physiology, evolution and ecology. New Phytol. 2017, 213, 1582–1596. [Google Scholar] [CrossRef]
- Brozmanova, J.; Manikova, D.; Vlckova, V.; Chovanec, M. Selenium: A double-edged sword for defense and offence in cancer. Arch. Toxicol. 2010, 84, 919–938. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009; Available online: http://www.who.int/iris/handle/10665/44203 (accessed on 16 June 2012).
- Vinceti, M.; Filippini, T.; Cilloni, S.; Bargellini, A.; Vergoni, A.V.; Tsatsakis, A.; Ferrante, M. Health risk assessment of environmental selenium: Emerging evidence and challenges (Review). Mol. Med. Rep. 2017, 15, 3323–3335. [Google Scholar] [CrossRef]
- Fordyce, F.M. Selenium Deficiency and Toxicity in the Environment Essentials of Medical Geology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 375–416. [Google Scholar]
- Ahmad, P.; Allah, E.F.A.; Hashem, A.; Sarwat, M.; Gucel, S. Exogenous Application of Selenium Mitigates Cadmium Toxicity in Brassica juncea L. (Czern & Cross) by Up-Regulating Antioxidative System and Secondary Metabolites. J. Plant. Growth Regul. 2016, 35, 936–950. [Google Scholar]
- Liu, W.; Shang, S.; Feng, X.; Zhang, G.; Wu, F. Modulation of exogenous selenium in cadmium-induced changes in antioxidative metabolism, cadmium uptake, and photosynthetic performance in the 2 tobacco genotypes differing in cadmium tolerance. Environ. Toxicol. Chem. 2015, 34, 92–99. [Google Scholar] [CrossRef]
- Huang, X.; Yang, S.; Gong, J.; Zhao, Q.; Feng, Q.; Zhan, Q.; Zhao, Y.; Li, W.; Cheng, B.; Xia, J.; et al. Genomic architecture of heterosis for yield traits in rice. Nature 2016, 537, 629–633. [Google Scholar] [CrossRef]
- Liang, Y.; Farooq, M.U.; Zeng, R.; Tang, Z.; Zhang, Y.; Zheng, T.; Ei, H.H.; Ye, X.; Jia, X.; Zhu, J. Breeding of selenium rich red glutinous rice, protein extraction and analysis of the distribution of selenium in grain. Int. J. Agric. Biol. 2018, 20, 1005–1011. [Google Scholar]
- Farooq, M.U.; Tang, Z.; Zeng, R.; Liang, Y.; Zhang, Y.; Zheng, T.; Ei, H.H.; Ye, X.; Jia, X.; Zhu, J. Accumulation, mobilization, and transformation of selenium in rice grain provided with foliar sodium selenite. J. Sci. Food Agric. 2019, 99, 2892–2900. [Google Scholar] [CrossRef]
- Farooq, M.U.; Khan, A.; Ishaaq, I.; Cheema, A.; Afzal, M.; Ali, A.; Zhu, J. Growing Degree Days during the Late Reproductive Phase Determine Spike Density and Cognate Yield Traits. Agronomy 2018, 8, 217. [Google Scholar] [CrossRef]
- Peng, K.; Luo, C.; Chen, Y.; Wang, G.; Li, X.; Shen, Z. Cadmium and other metal uptake by Lobelia chinensis and Solanum nigrum from contaminated soils. Bull. Environ. Cont. Toxicol. 2009, 83, 260–264. [Google Scholar] [CrossRef]
- Clemens, S.; Aarts, M.G.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant. Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012, 37. [Google Scholar] [CrossRef]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef]
- Li, L.; Wu, H.; van Gestel, C.A.; Peijnenburg, W.J.; Allen, H.E. Soil acidification increases metal extractability and bioavailability in old orchard soils of Northeast Jiaodong Peninsula in China. Environ. Pollut. 2014, 188, 144–152. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Liu, C.; Zhu, J.; Li, F.; Deng, D.-M.; Wang, Q.; Liu, C. Cadmium availability in rice paddy fields from a mining area: The effects of soil properties highlighting iron fractions and pH value. Environ. Pollut. 2016, 209, 38–45. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant. Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Duan, Y.; Fu, T.; Fu, J. Studies on the biological accumulation and antioxidation of selenium in garlic (Allium sativum L.). Acta Horticul. Sin. 1997, 24, 343–347. [Google Scholar]
- Li, X.; Wu, Y.; Li, B.; Yang, Y.; Yang, Y. Selenium accumulation characteristics and biofortification potentiality in Turnip (Brassica rapa var. rapa) Supplied with selenite or selenate. Front. Plant. Sci. 2018, 8, 2207. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, Y.; Liu, Y.; Zeng, G.; Hu, X.; Hu, X.; Zhou, L.; Guo, Y.; Li, J. Cadmium accumulation and apoplastic and symplastic transport in Boehmeria nivea (L.) Gaudich on cadmium-contaminated soil with the addition of EDTA or NTA. Rsc. Adv. 2015, 5, 47584–47591. [Google Scholar] [CrossRef]
- Lux, A.; Martinka, M.; Vaculík, M.; White, P.J. Root responses to cadmium in the rhizosphere: A review. J. Exp. Bot. 2010, 62, 21–37. [Google Scholar] [CrossRef]
- Salt, D.E.; Prince, R.C.; Pickering, I.J.; Raskin, I. Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol. 1995, 109, 1427–1433. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-Ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Yong, S.O. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 1–21. [Google Scholar] [CrossRef]
- Luo, J.-S.; Huang, J.; Zeng, D.-L.; Peng, J.-S.; Zhang, G.-B.; Ma, H.-L.; Guan, Y.; Yi, H.-Y.; Fu, Y.-L.; Han, B. A defensin-like protein drives cadmium efflux and allocation in rice. Nat. Commun. 2018, 9, 645. [Google Scholar] [CrossRef]
- Uraguchi, S.; Mori, S.; Kuramata, M.; Kawasaki, A.; Arao, T.; Ishikawa, S. Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J. Exp. Bot. 2009, 60, 2677–2688. [Google Scholar] [CrossRef] [Green Version]
- Jinadasa, N.; Collins, D.; Holford, P.; Milham, P.J.; Conroy, J.P. Reactions to cadmium stress in a cadmium-tolerant variety of cabbage (Brassica oleracea L.): Is cadmium tolerance necessarily desirable in food crops? Environ. Sci. Pollut. Res. 2016, 23, 5296–5306. [Google Scholar] [CrossRef]
- Ming, Z.C.; Fan, L.; Gao, A.; Zhou, X. Absorption, Translocation and Redistribution of Selenium Supplied at Different Growth Stages of Rice. Int. J. Agric. Biol. 2017, 19, 1601–1607. [Google Scholar]
- Zhao, J. Effects of Selenium on the Biological Behavior and Effects of Mercury in Plants. Master’s Thesis, University of Chinese Academy of Sciences, Beijing, China, 2013. [Google Scholar]
- Wang, M. Caryopsis Development of Rice. Ph.D. Thesis, Yangzhou University, Yangzhou, China, 2011. [Google Scholar]
- Zhai, G. Study on the Development of Amyloplast in Rice Endosperm Cells. Ph.D. Thesis, Yangzhou University, Yangzhou, China, 2012. [Google Scholar]
- Jacobsen, S.; Olszewski, N. Gibberellins regulate the abundance of RNAs with sequence similarity to proteinase inhibitors, dioxygenases and dehydrogenases. Planta 1996, 198, 78–86. [Google Scholar] [CrossRef]
- Chi, F.Q.; Kuang, E.J.; Zhang, J.M.; Su, Q.R.; Wei, D. Effect of different selenium fertilizer application methods and application periods on selenium content and yield of rice. J. Agric. Res. Environ. 2014, 6, 81–107. [Google Scholar]
- Zhu, W.D. Application of Selenium on Foliar Surface of Rice Could Enhance the Percentage of Selenium and Improve Quality of Rice. Hubei Agric. Sci. 2013, 52, 1764–1767. [Google Scholar]
- Teng, S.; Li, X.; Cao, H.; Liu, S.; Li, Z. Effect of Foliar Application of Selenium Fertilizer on the Yield and Selenium Content in Rice. J. Agric. 2015, 5, 1–3. [Google Scholar]
- Upadhyaya, H.; Panda, S.K.; Bhattacharjee, M.K.; Dutta, S. Role of arbuscular mycorrhiza in heavy metal tolerance in plants: Prospects for phytoremidiation. J. Phytol. 2010, 2, 16–27. [Google Scholar]
- Bañuelos, G. Selenium in the Environment and Human Health; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Satarug, S.; Moore, M.R. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ. Health Persp. 2004, 112, 1099–1103. [Google Scholar] [CrossRef]
- Yao, X.; Chu, J.; Wang, G. Effects of selenium on wheat seedlings under drought stress. Biol. Trace Elem. Res. 2009, 130, 283–290. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Ge, L.; Cang, L.; Yang, J.; Zhou, D. Effects of root morphology and leaf transpiration on Cd uptake and translocation in rice under different growth temperature. Environ. Sci. Pollut. Res. 2016, 23, 24205–24214. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.-Q.; Cang, L.; Liu, H.; Zhou, D.-M. Effects of warming on uptake and translocation of cadmium (Cd) and copper (Cu) in a contaminated soil-rice system under Free Air Temperature Increase (FATI). Chemosphere 2016, 155, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.U.; Zhu, J. The paradox in accumulation behavior of cadmium and selenium at different planting times in rice. Environ. Sci. Pollut. Res. 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Laza, M.R.C.; Sakai, H.; Cheng, W.; Tokida, T.; Peng, S.; Hasegawa, T. Differential response of rice plants to high night temperatures imposed at varying developmental phases. Agric. For. Meterol. 2015, 209–210, 69–77. [Google Scholar] [CrossRef]
- Premarathna, L.; McLaughlin, M.J.; Kirby, J.K.; Hettiarachchi, G.M.; Stacey, S.; Chittleborough, D.J. Selenate-enriched urea granules are a highly effective fertilizer for selenium biofortification of paddy rice grain. J. Agric. Food Chem. 2012, 60, 6037–6044. [Google Scholar] [CrossRef] [PubMed]



| Index | Contents (mg kg−1 Dry Soil) |
|---|---|
| pH | 5.98 |
| OM | 32,870 |
| Total N | 172 |
| Total P | 1960 |
| Total Se | 0.3242 |
| Total Cd | 0.0912 |
| Treatment | Na2SeO3 (mg kg−1 Dry Soil) | CdCl2.2½H2O (mg kg−1 Dry Soil) | Remarks |
|---|---|---|---|
| T0 (Control) | - | - | Adequate all |
| T1 | 0.4 | 1 | Medium Stress |
| T2 | 1 | 2 | High Stress |
| Interaction Effects | Soil | Roots | Stem | Leaves | Panicle | Panicle Straw | Husk | Rice Bran | Embryo | Endosperm | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Varieties vs. Stress | |||||||||||
| V1 × Se | 0.220 b | 2.090 b | 1.039 a | 0.689 a | 3.399 a | 0.791 a | 0.971 a | 1.046 a | 0.458 abc | 0.132 a | |
| V2 × Se | 0.914 ab | 3.236 a | 1.018 a | 0.975 a | 2.968 a | 0.525 ab | 0.658 ab | 0.820 a | 0.608 ab | 0.355 a | |
| V3 × Se | 0.738 ab | 2.969 ab | 1.033 a | 0.962 a | 3.068 a | 0.505 ab | 0.669 ab | 0.855 a | 0.666 a | 0.370 a | |
| V1 × Cd | 2.428 a | 0.277 c | 0.190 b | 0.155 a | 0.369 c | 0.092 b | 0.085 c | 0.106 b | 0.044 c | 0.041 a | |
| V2 × Cd | 1.887 ab | 0.674 c | 0.152 b | 0.199 a | 1.080 b | 0.225 b | 0.296 bc | 0.281 b | 0.146 bc | 0.131 a | |
| V3 × Cd | 1.969 a | 0.561 c | 0.133 b | 0.152 a | 1.004 bc | 0.181 b | 0.266 bc | 0.288 b | 0.153 bc | 0.115 a | |
| Treatments vs. Stress | |||||||||||
| T0 × Se | 0.288 b | 0.517 c | 0.218 b | 0.167 b | 0.695 bc | 0.127 b | 0.135 b | 0.254 bc | 0.135 b | 0.043 b | |
| T0 × Cd | 0.230 b | 0.277 c | 0.121 b | 0.110 b | 0.480 c | 0.126 b | 0.128 b | 0.101 c | 0.066 b | 0.058 b | |
| T1 × Se | 0.474 b | 2.433 b | 0.284 b | 0.212 b | 1.197 b | 0.190 b | 0.262 b | 0.576 b | 0.124 b | 0.045 b | |
| T1 × Cd | 0.935 b | 0.674 c | 0.240 b | 0.213 b | 1.040 bc | 0.204 b | 0.289 b | 0.393 bc | 0.079 b | 0.074 b | |
| T2 × Se | 1.110 b | 5.334 a | 2.588 a | 2.246 a | 7.543 a | 1.505 a | 1.902 a | 1.892 a | 1.473 a | 0.769 a | |
| T2 × Cd | 5.123 a | 0.561 c | 0.113 b | 0.184 b | 0.933 bc | 0.168 b | 0.231 b | 0.181 bc | 0.197 b | 0.155 b | |
| S. E | 0.6270 | 0.3379 | 0.2791 | 0.3777 | 0.2391 | 0.2015 | 0.1917 | 0.1688 | 0.1737 | 0.1548 | |
| LSD (0.05) | 1.7408 | 0.9383 | 0.7748 | 1.0487 | 0.6638 | 0.5595 | 0.5321 | 0.4685 | 0.4822 | 0.4299 | |
| Interaction Effects | |||||||||||
| (Varieties × Stress) | * | * | * | ns | * | * | * | * | * | ns | |
| (Treatment × Stress) | * | * | * | * | * | * | * | * | * | * | |
| Estimated Date of the Respective Stage | |||||||||
| Varieties | Sowing | Transplanting | Stress Treatment | Tillering | 50% Heading | Flowering | 50% Maturity | Maturity | Harvesting |
| 5097A/R2035 | 13/04 | 23/05 | 03/06 | 22/07 | 14/08 | 21/08 | 27/08 | 04/09 | 12/09 |
| GangYou 725 | 13/04 | 23/05 | 04/06 | 18/07 | 08/08 | 14/08 | 21/08 | 31/08 | 06/09 |
| 2057A/R881 | 13/04 | 23/05 | 04/06 | 28/07 | 16/08 | 22/08 | 28/08 | 05/09 | 11/09 |
| Days took to achieve the respective stage | |||||||||
| Transplanting | Stress Treatment | Tillering | 50% Heading | Flowering | 50% Maturity | Maturity | Harvesting | ||
| 5097A/R2035 | -- | 40 | 51 | 95 | 116 | 122 | 129 | 139 | 145 |
| GangYou 725 | -- | 40 | 50 | 99 | 122 | 129 | 135 | 143 | 151 |
| 2057A/R881 | -- | 40 | 51 | 105 | 124 | 130 | 136 | 144 | 150 |
| Heat units consumed by rice to achieve the respective stage | |||||||||
| Transplanting | Stress Treatment | Tillering | 50% Heading | Flowering | 50% Maturity | Maturity | Harvesting | ||
| 5097A/R2035 | -- | 408 | 563 | 1224 | 1598.5 | 1702.5 | 1818.5 | 1981.5 | 2054.5 |
| GangYou 725 | -- | 408 | 550.5 | 1292.5 | 1702.5 | 1818.5 | 1916.5 | 2031 | 2142 |
| 2057A/R881 | -- | 408 | 563 | 1398 | 1736 | 1836 | 1938 | 2042 | 2126.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, M.U.; Tang, Z.; Zheng, T.; Asghar, M.A.; Zeng, R.; Su, Y.; Ei, H.H.; Liang, Y.; Zhang, Y.; Ye, X.; et al. Cross-Talk between Cadmium and Selenium at Elevated Cadmium Stress Determines the Fate of Selenium Uptake in Rice. Biomolecules 2019, 9, 247. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9060247
Farooq MU, Tang Z, Zheng T, Asghar MA, Zeng R, Su Y, Ei HH, Liang Y, Zhang Y, Ye X, et al. Cross-Talk between Cadmium and Selenium at Elevated Cadmium Stress Determines the Fate of Selenium Uptake in Rice. Biomolecules. 2019; 9(6):247. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9060247
Chicago/Turabian StyleFarooq, Muhammad Umer, Zhichen Tang, Tengda Zheng, Muhammad Ahsan Asghar, Rui Zeng, Yang Su, Hla Hla Ei, Yuanke Liang, Yujie Zhang, Xiaoying Ye, and et al. 2019. "Cross-Talk between Cadmium and Selenium at Elevated Cadmium Stress Determines the Fate of Selenium Uptake in Rice" Biomolecules 9, no. 6: 247. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9060247