The Role of Distinctive Sphingolipids in the Inflammatory and Apoptotic Effects of Electronegative LDL on Monocytes
Abstract
:1. Introduction
2. Material and Methods
2.1. LDL Isolation and Modification
2.2. Lipidomic Analysis
2.3. Inflammatory Action on Monocytes
2.4. Apoptosis Assay
2.5. Statistical Analyses
3. Results
3.1. LDL(−) Has an Increased Content in Sph and Cer
3.2. Sph and Cer Are Involved in the Cytokine Release Promoted by LDL(−) in Monocytes
3.3. Cer Is Involved in LDL(−)-Induced Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edsfeldt, A.; Duner, P.; Stahlman, M.; Mollet, I.G.; Asciutto, G.; Grufman, H.; Nitulescu, M.; Persson, A.F.; Fisher, R.M.; Melander, O.; et al. Sphingolipids contribute to human atherosclerotic plaque inflammation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Obeid, L.M. Ceramidases: Regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim. Biophys. Acta 2008, 1781, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Arana, L.; Gangoiti, P.; Ouro, A.; Trueba, M.; Gomez-Munoz, A. Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis. 2010, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Havulinna, A.S.; Sysi-Aho, M.; Hilvo, M.; Kauhanen, D.; Hurme, R.; Ekroos, K.; Salomaa, V.; Laaksonen, R. Circulating ceramides predict cardiovascular outcomes in the population-based finrisk 2002 cohort. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2424–2430. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, J.W.; Donato, L.J.; Bryant, S.C.; Baudhuin, L.M.; Berger, P.B.; Jaffe, A.S. Plasma ceramides. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, J. Sphingosine and ceramide signalling in apoptosis. IUBMB Life 2006, 58, 462–466. [Google Scholar] [CrossRef]
- Luheshi, N.M.; Giles, J.A.; Lopez-Castejon, G.; Brough, D. Sphingosine regulates the nlrp3-inflammasome and il-1beta release from macrophages. Eur. J. Immunol. 2012, 42, 716–725. [Google Scholar] [CrossRef]
- Suzuki, E.; Handa, K.; Toledo, M.S.; Hakomori, S. Sphingosine-dependent apoptosis: A unified concept based on multiple mechanisms operating in concert. Proc. Natl. Acad. Sci. USA 2004, 101, 14788–14793. [Google Scholar] [CrossRef] [Green Version]
- Monick, M.M.; Cameron, K.; Powers, L.S.; Butler, N.S.; McCoy, D.; Mallampalli, R.K.; Hunninghake, G.W. Sphingosine kinase mediates activation of extracellular signal-related kinase and akt by respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 2004, 30, 844–852. [Google Scholar] [CrossRef]
- Muller, J.; von Bernstorff, W.; Heidecke, C.D.; Schulze, T. Differential s1p receptor profiles on m1- and m2-polarized macrophages affect macrophage cytokine production and migration. Biomed. Res. Int. 2017, 2017, 7584621. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Harikumar, K.B.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012, 22, 50–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Coso, O.A.; Gutkind, S.; Spiegel, S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 1996, 381, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.; Leung, B.P.; Melendez, A.J. Sphingosine kinase 1 regulates pro-inflammatory responses triggered by tnfalpha in primary human monocytes. J. Cell Physiol. 2006, 208, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.Q.; Irwan, A.W.; Goh, H.H.; Howe, H.S.; Yu, D.T.; Valle-Onate, R.; McInnes, I.B.; Melendez, A.J.; Leung, B.P. Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis. J. Immunol. 2008, 181, 8010–8017. [Google Scholar] [CrossRef]
- Jin, J.; Lu, Z.; Li, Y.; Ru, J.H.; Lopes-Virella, M.F.; Huang, Y. Lps and palmitate synergistically stimulate sphingosine kinase 1 and increase sphingosine 1 phosphate in raw264.7 macrophages. J. Leukoc. Biol. 2018, 104, 843–853. [Google Scholar] [CrossRef]
- Ruiz, M.; Frej, C.; Holmer, A.; Guo, L.J.; Tran, S.; Dahlback, B. High-density lipoprotein-associated apolipoprotein m limits endothelial inflammation by delivering sphingosine-1-phosphate to the sphingosine-1-phosphate receptor 1. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 118–129. [Google Scholar] [CrossRef]
- Poti, F.; Simoni, M.; Nofer, J.R. Atheroprotective role of high-density lipoprotein (hdl)-associated sphingosine-1-phosphate (s1p). Cardiovasc. Res. 2014, 103, 395–404. [Google Scholar] [CrossRef]
- Sattler, K.; Levkau, B. Sphingosine-1-phosphate as a mediator of high-density lipoprotein effects in cardiovascular protection. Cardiovasc. Res. 2009, 82, 201–211. [Google Scholar] [CrossRef]
- Hammad, S.M.; Pierce, J.S.; Soodavar, F.; Smith, K.J.; Al Gadban, M.M.; Rembiesa, B.; Klein, R.L.; Hannun, Y.A.; Bielawski, J.; Bielawska, A. Blood sphingolipidomics in healthy humans: Impact of sample collection methodology. J. Lipid Res. 2010, 51, 3074–3087. [Google Scholar] [CrossRef] [PubMed]
- Estruch, M.; Sanchez-Quesada, J.L.; Beloki, L.; Ordonez-Llanos, J.; Benitez, S. The induction of cytokine release in monocytes by electronegative low-density lipoprotein (ldl) is related to its higher ceramide content than native ldl. Int. J. Mol. Sci. 2013, 14, 2601–2616. [Google Scholar] [CrossRef] [PubMed]
- Boon, J.; Hoy, A.J.; Stark, R.; Brown, R.D.; Meex, R.C.; Henstridge, D.C.; Schenk, S.; Meikle, P.J.; Horowitz, J.F.; Kingwell, B.A.; et al. Ceramides contained in ldl are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 2013, 62, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Estruch, M.; Sanchez-Quesada, J.L.; Ordonez Llanos, J.; Benitez, S. Electronegative ldl: A circulating modified ldl with a role in inflammation. Mediators Inflamm. 2013, 2013, 181324. [Google Scholar] [CrossRef] [PubMed]
- Benitez, S.; Sanchez-Quesada, J.L.; Ribas, V.; Jorba, O.; Blanco-Vaca, F.; Gonzalez-Sastre, F.; Ordonez-Llanos, J. Platelet-activating factor acetylhydrolase is mainly associated with electronegative low-density lipoprotein subfraction. Circulation 2003, 108, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Bancells, C.; Benitez, S.; Villegas, S.; Jorba, O.; Ordonez-Llanos, J.; Sanchez-Quesada, J.L. Novel phospholipolytic activities associated with electronegative low-density lipoprotein are involved in increased self-aggregation. Biochemistry 2008, 47, 8186–8194. [Google Scholar] [CrossRef] [PubMed]
- Estruch, M.; Sanchez-Quesada, J.L.; Ordonez-Llanos, J.; Benitez, S. Ceramide-enriched ldl induces cytokine release through tlr4 and cd14 in monocytes. Similarities with electronegative ldl. Clin. Investig. Arterioscler. 2014, 26, 131–137. [Google Scholar] [CrossRef]
- Bancells, C.; Sanchez-Quesada, J.L.; Birkelund, R.; Ordonez-Llanos, J.; Benitez, S. Hdl and electronegative ldl exchange anti- and pro-inflammatory properties. J. Lipid Res. 2010, 51, 2947–2956. [Google Scholar] [CrossRef]
- Pedrosa, A.M.; Faine, L.A.; Grosso, D.M.; de Las Heras, B.; Bosca, L.; Abdalla, D.S. Electronegative ldl induction of apoptosis in macrophages: Involvement of nrf2. Biochim. Biophys. Acta 2010, 1801, 430–437. [Google Scholar] [CrossRef]
- Ke, L.Y.; Chan, H.C.; Chen, C.C.; Lu, J.; Marathe, G.K.; Chu, C.S.; Wang, C.Y.; Tung, Y.C.; McIntyre, T.M.; Yen, J.H.; et al. Enhanced sphingomyelinase activity contributes to the apoptotic capacity of electronegative low-density lipoprotein. J. Med. Chem. 2016, 59, 1032–1040. [Google Scholar] [CrossRef]
- Deigner, H.P.; Claus, R.; Bonaterra, G.A.; Gehrke, C.; Bibak, N.; Blaess, M.; Cantz, M.; Metz, J.; Kinscherf, R. Ceramide induces asmase expression: Implications for oxldl-induced apoptosis. FASEB J. 2001, 15, 807–814. [Google Scholar] [CrossRef]
- Artwohl, M.; Roden, M.; Waldhausl, W.; Freudenthaler, A.; Baumgartner-Parzer, S.M. Free fatty acids trigger apoptosis and inhibit cell cycle progression in human vascular endothelial cells. FASEB J. 2004, 18, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Boyanovsky, B.; Karakashian, A.; King, K.; Giltiay, N.; Nikolova-Karakashian, M. Uptake and metabolism of low density lipoproteins with elevated ceramide content by human microvascular endothelial cells: Implications for the regulation of apoptosis. J. Biol. Chem. 2003, 278, 26992–26999. [Google Scholar] [CrossRef] [PubMed]
- Dersch, K.; Ichijo, H.; Bhakdi, S.; Husmann, M. Fatty acids liberated from low-density lipoprotein trigger endothelial apoptosis via mitogen-activated protein kinases. Cell Death Differ. 2005, 12, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Loidl, A.; Claus, R.; Ingolic, E.; Deigner, H.P.; Hermetter, A. Role of ceramide in activation of stress-associated map kinases by minimally modified ldl in vascular smooth muscle cells. Biochim. Biophys. Acta 2004, 1690, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Estruch, M.; Rajamaki, K.; Sanchez-Quesada, J.L.; Kovanen, P.T.; Oorni, K.; Benitez, S.; Ordonez-Llanos, J. Electronegative ldl induces priming and inflammasome activation leading to il-1beta release in human monocytes and macrophages. Biochim. Biophys. Acta 2015, 1851, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Benitez, S.; Bancells, C.; Ordonez-Llanos, J.; Sanchez-Quesada, J.L. Pro-inflammatory action of ldl(-) on mononuclear cells is counteracted by increased il10 production. Biochim. Biophys. Acta 2007, 1771, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Estruch, M.; Sanchez-Quesada, J.L.; Ordonez-Llanos, J.; Benitez, S. Inflammatory intracellular pathways activated by electronegative ldl in monocytes. Biochim. Biophys. Acta 2016, 1861, 963–969. [Google Scholar] [CrossRef]
- Damirin, A.; Tomura, H.; Komachi, M.; Liu, J.P.; Mogi, C.; Tobo, M.; Wang, J.Q.; Kimura, T.; Kuwabara, A.; Yamazaki, Y.; et al. Role of lipoprotein-associated lysophospholipids in migratory activity of coronary artery smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2513–2522. [Google Scholar] [CrossRef]
- Obinata, H.; Hla, T. Sphingosine 1-phosphate in coagulation and inflammation. Semin. Immunopathol. 2012, 34, 73–91. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chen, H.H.; Huang, M.T.; Raya, J.L.; Yang, J.H.; Chen, C.H.; Gaubatz, J.W.; Pownall, H.J.; Taylor, A.A.; Ballantyne, C.M.; et al. Pro-apoptotic low-density lipoprotein subfractions in type ii diabetes. Atherosclerosis 2007, 193, 283–291. [Google Scholar] [CrossRef]
- Lu, J.; Yang, J.H.; Burns, A.R.; Chen, H.H.; Tang, D.; Walterscheid, J.P.; Suzuki, S.; Yang, C.Y.; Sawamura, T.; Chen, C.H. Mediation of electronegative low-density lipoprotein signaling by lox-1: A possible mechanism of endothelial apoptosis. Circ. Res. 2009, 104, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Bancells, C.; Sanchez-Quesada, J.L.; Birkelund, R.; Ordonez-Llanos, J.; Benitez, S. Electronegative ldl induces fas and modifies gene expression in mononuclear cells. Front. Biosci. (Elite Ed.) 2010, 2, 78–86. [Google Scholar] [PubMed]
- Seimon, T.A.; Nadolski, M.J.; Liao, X.; Magallon, J.; Nguyen, M.; Feric, N.T.; Koschinsky, M.L.; Harkewicz, R.; Witztum, J.L.; Tsimikas, S.; et al. Atherogenic lipids and lipoproteins trigger cd36-tlr2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010, 12, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Namgaladze, D.; Kollas, A.; Brune, B. Oxidized ldl attenuates apoptosis in monocytic cells by activating erk signaling. J. Lipid Res. 2008, 49, 58–65. [Google Scholar]
- Baird, S.K.; Hampton, M.B.; Gieseg, S.P. Oxidized ldl triggers phosphatidylserine exposure in human monocyte cell lines by both caspase-dependent and -independent mechanisms. FEBS Lett. 2004, 578, 169–174. [Google Scholar]
- Jostarndt, K.; Gellert, N.; Rubic, T.; Weber, C.; Kuhn, H.; Johansen, B.; Hrboticky, N.; Neuzil, J. Dissociation of apoptosis induction and cd36 upregulation by enzymatically modified low-density lipoprotein in monocytic cells. Biochem. Biophys. Res. Commun. 2002, 290, 988–993. [Google Scholar]
- Lu, J.; Jiang, W.; Yang, J.H.; Chang, P.Y.; Walterscheid, J.P.; Chen, H.H.; Marcelli, M.; Tang, D.; Lee, Y.T.; Liao, W.S.; et al. Electronegative ldl impairs vascular endothelial cell integrity in diabetes by disrupting fibroblast growth factor 2 (fgf2) autoregulation. Diabetes 2008, 57, 158–166. [Google Scholar]
- Chen, C.H.; Jiang, T.; Yang, J.H.; Jiang, W.; Lu, J.; Marathe, G.K.; Pownall, H.J.; Ballantyne, C.M.; McIntyre, T.M.; Henry, P.D.; et al. Low-density lipoprotein in hypercholesterolemic human plasma induces vascular endothelial cell apoptosis by inhibiting fibroblast growth factor 2 transcription. Circulation 2003, 107, 2102–2108. [Google Scholar]
- Benitez, S.; Camacho, M.; Arcelus, R.; Vila, L.; Bancells, C.; Ordonez-Llanos, J.; Sanchez-Quesada, J.L. Increased lysophosphatidylcholine and non-esterified fatty acid content in ldl induces chemokine release in endothelial cells. Relationship with electronegative ldl. Atherosclerosis 2004, 177, 299–305. [Google Scholar]
- Carpenter, K.L.; Dennis, I.F.; Challis, I.R.; Osborn, D.P.; Macphee, C.H.; Leake, D.S.; Arends, M.J.; Mitchinson, M.J. Inhibition of lipoprotein-associated phospholipase a2 diminishes the death-inducing effects of oxidised ldl on human monocyte-macrophages. FEBS Lett. 2001, 505, 357–363. [Google Scholar]










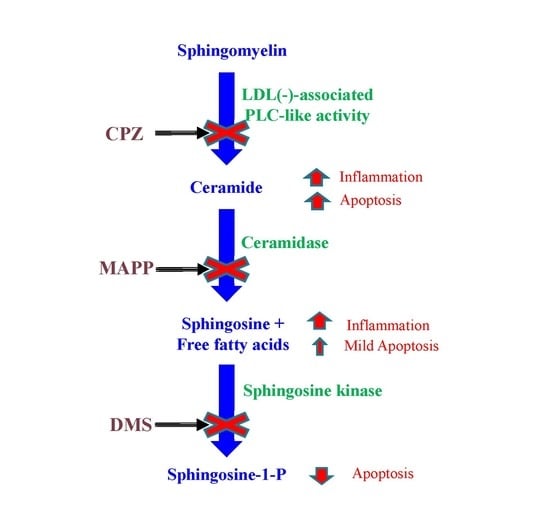
| Inhibitor | Abbreviation | Enzyme | Substrate | Product |
|---|---|---|---|---|
| Chlorpromazine | CPZ | SMase | SM | Cer and phosphorylcholine |
| d-erythro-2-(N-myristoyl amino)-1-phenyl-1-propanol | MAPP | CDase | Cer | Sph and NEFA |
| N,N-dimethylsphingosine | DMS | Sph kinase | Sph | S1P |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puig, N.; Estruch, M.; Jin, L.; Sanchez-Quesada, J.L.; Benitez, S. The Role of Distinctive Sphingolipids in the Inflammatory and Apoptotic Effects of Electronegative LDL on Monocytes. Biomolecules 2019, 9, 300. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9080300
Puig N, Estruch M, Jin L, Sanchez-Quesada JL, Benitez S. The Role of Distinctive Sphingolipids in the Inflammatory and Apoptotic Effects of Electronegative LDL on Monocytes. Biomolecules. 2019; 9(8):300. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9080300
Chicago/Turabian StylePuig, Núria, Montserrat Estruch, Lei Jin, Jose Luis Sanchez-Quesada, and Sonia Benitez. 2019. "The Role of Distinctive Sphingolipids in the Inflammatory and Apoptotic Effects of Electronegative LDL on Monocytes" Biomolecules 9, no. 8: 300. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9080300