Solid Magnetoliposomes as Multi-Stimuli-Responsive Systems for Controlled Release of Doxorubicin: Assessment of Lipid Formulations
Abstract
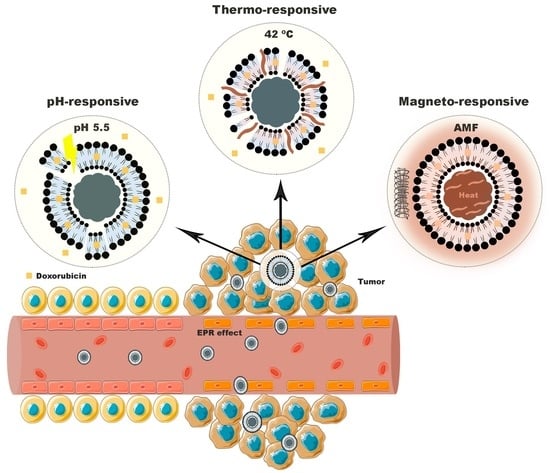
:1. Introduction
2. Materials and Methods
2.1. Solid Magnetoliposomes Preparation
2.2. Magnetoliposomes Characterization
2.2.1. Dynamic Light Scattering Measurements
2.2.2. Fluorescence Spectroscopy Measurements
2.2.3. Differential Scanning Calorimetry
3. Results and Discussion
3.1. Influence of Formulation on Structural, Colloidal and Thermodynamic Parameters of DOX-Loaded SMLs
3.2. Interaction with HSA
3.3. Stability of Liposomal Aqueous Suspensions upon Storage at 4 °C
3.4. Implications of Lipid Formulation and pH on Location of DOX
3.5. Effect of Lipid Formulations on DOX Release Kinetics and Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathers, C.D. History of global burden of disease assessment at the World Health Organization. Arch. Public Health 2020, 78, 77. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, T.O.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyas, D.; Laput, G.; Vyas, A.K. Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. OncoTargets Ther. 2014, 7, 1015. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin nephrotoxicity: A review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Von Hoff, D.D.; Mita, M.M.; Ramanathan, R.K.; Weiss, G.J.; Mita, A.C.; LoRusso, P.M.; Burris, H.A.; Hart, L.L.; Low, S.C.; Parsons, D.M. Phase I study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin. Cancer Res. 2016, 22, 3157–3163. [Google Scholar] [CrossRef] [Green Version]
- Barenholz, Y.C. Doxil®—the first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Mross, K.; Niemann, B.; Massing, U.; Drevs, J.; Unger, C.; Bhamra, R.; Swenson, C.E. Pharmacokinetics of liposomal doxorubicin (TLC-D99; Myocet) in patients with solid tumors: An open-label, single-dose study. Cancer Chemother. Pharmacol. 2004, 54, 514–524. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Arap, W.; Pasqualini, R.; Ruoslahti, E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998, 279, 377–380. [Google Scholar] [CrossRef] [Green Version]
- Motamarry, A.; Asemani, D.; Haemmerich, D. Thermosensitive liposomes. In Liposomes; InTech: Rijeka, Croatia, 2017; pp. 187–212. [Google Scholar] [CrossRef] [Green Version]
- Yatvin, M.B.; Weinstein, J.N.; Dennis, W.H.; Blumenthal, R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science 1978, 202, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Oude Blenke, E.; Mastrobattista, E.; Schiffelers, R.M. Strategies for triggered drug release from tumor targeted liposomes. Expert Opin. Drug Deliv. 2013, 10, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T.; Lawrence, T.S.; Rosenberg, S.A. DeVita, Hellman, and Rosenberg's Cancer: Principles & Practice of Oncology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; Volume 2. [Google Scholar]
- Jha, S.; Sharma, P.K.; Malviya, R. Hyperthermia: Role and risk factor for cancer treatment. Achiev. Life Sci. 2016, 10, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Datta, N.; Ordóñez, S.G.; Gaipl, U.; Paulides, M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef]
- Mantso, T.; Vasileiadis, S.; Anestopoulos, I.; Voulgaridou, G.-P.; Lampri, E.; Botaitis, S.; Kontomanolis, E.; Simopoulos, C.; Goussetis, G.; Franco, R. Hyperthermia induces therapeutic effectiveness and potentiates adjuvant therapy with non-targeted and targeted drugs in an in vitro model of human malignant melanoma. Sci. Rep. 2018, 8, 10724. [Google Scholar] [CrossRef]
- Van der Heijden, A.G.; Dewhirst, M.W. Effects of hyperthermia in neutralising mechanisms of drug resistance in non-muscle-invasive bladder cancer. Int. J. Hyperth. 2016, 32, 434–445. [Google Scholar] [CrossRef] [Green Version]
- Kampinga, H.H. Cell biological effects of hyperthermia alone or combined with radiation or drugs: A short introduction to newcomers in the field. Int. J. Hyperth. 2006, 22, 191–196. [Google Scholar] [CrossRef]
- Song, C.W. Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Res. 1984, 44, 4721s–4730s. [Google Scholar]
- Chang, D.; Lim, M.; Goos, J.A.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically targeted magnetic hyperthermia: Potential and limitations. Front. Pharmacol. 2018, 9, 831. [Google Scholar] [CrossRef] [Green Version]
- Gavilán, H.; Avugadda, S.K.; Fernández-Cabada, T.; Soni, N.; Cassani, M.; Mai, B.T.; Chantrell, R.; Pellegrino, T. Magnetic nanoparticles and clusters for magnetic hyperthermia: Optimizing their heat performance and developing combinatorial therapies to tackle cancer. Chem. Soc. Rev. 2021, 50, 11614–11667. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yan, C.; Yan, Y.; Chen, L.; Song, L.; Zang, F.; An, Y.; Teng, G.; Gu, N.; Zhang, Y. Multi-modal Mn–Zn ferrite nanocrystals for magnetically-induced cancer targeted hyperthermia: A comparison of passive and active targeting effects. Nanoscale 2016, 8, 16902–16915. [Google Scholar] [CrossRef] [PubMed]
- Yatvin, M.; Kreutz, W.; Horwitz, B.; Shinitzky, M. pH-Sensitive Liposomes: Possible Clinical Implications. Science 1980, 210, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.S.; Gomes, E.R.; Roque, M.C.; Oliveira, M.C. Triggered drug release from liposomes: Exploiting the outer and inner tumor environment. Front. Oncol. 2021, 11, 470. [Google Scholar] [CrossRef]
- Karanth, H.; Murthy, R. pH-Sensitive liposomes-principle and application in cancer therapy. J. Pharm. Pharmacol. 2007, 59, 469–483. [Google Scholar] [CrossRef]
- Feng, L.; Dong, Z.; Tao, D.; Zhang, Y.; Liu, Z. The acidic tumor microenvironment: A target for smart cancer nano-theranostics. Natl. Sci. Rev. 2018, 5, 269–286. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, J.N.; Magin, R.; Yatvin, M.; Zaharko, D. Liposomes and local hyperthermia: Selective delivery of methotrexate to heated tumors. Science 1979, 204, 188–191. [Google Scholar] [CrossRef]
- Perrin, D. Buffers for pH and Metal Ion Control; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, B.D.; Rodrigues, A.R.O.; Bañobre-López, M.; Almeida, B.G.; Amorim, C.O.; Amaral, V.S.; Coutinho, P.J.; Castanheira, E. Magnetoliposomes based on shape anisotropic calcium/magnesium ferrite nanoparticles as nanocarriers for doxorubicin. Pharmaceutics 2021, 13, 1248. [Google Scholar] [CrossRef]
- Karukstis, K.K.; Thompson, E.H.; Whiles, J.A.; Rosenfeld, R.J. Deciphering the fluorescence signature of daunomycin and doxorubicin. Biophys. Chem. 1998, 73, 249–263. [Google Scholar] [CrossRef]
- Gijsbers, A.; Nishigaki, T.; Sánchez-Puig, N. Fluorescence anisotropy as a tool to study protein-protein interactions. JoVE J. Vis. Exp. 2016, 116, e54640. [Google Scholar] [CrossRef]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Chuang, V.T.G.; Maruyama, T.; Otagiri, M. Updates on contemporary protein binding techniques. Drug Metab. Pharmacokinet. 2009, 24, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.M.; Ribeiro, D.M.; Pinto, P.C.; Lúcio, M.; Reis, S.; Saraiva, M.L.M. Imidazolium ionic liquids as solvents of pharmaceuticals: Influence on HSA binding and partition coefficient of nimesulide. Int. J. Pharm. 2013, 443, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef] [Green Version]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Needham, D.; Dewhirst, M.W. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv. Drug Deliv. Rev. 2001, 53, 285–305. [Google Scholar] [CrossRef]
- Allen, T.M.; Hansen, C.; Rutledge, J. Liposomes with prolonged circulation times: Factors affecting uptake by reticuloendothelial and other tissues. Biochim. Biophys. Acta Biomembr. 1989, 981, 27–35. [Google Scholar] [CrossRef]
- Gaber, M.H.; Hong, K.; Huang, S.K.; Papahadjopoulos, D. Thermosensitive sterically stabilized liposomes: Formulation and in vitro studies on mechanism of doxorubicin release by bovine serum and human plasma. Pharm. Res. 1995, 12, 1407–1416. [Google Scholar] [CrossRef]
- Maruyama, K.; Unezaki, S.; Takahashi, N.; Iwatsuru, M. Enhanced delivery of doxorubicin to tumor by long-circulating thermosensitive liposomes and local hyperthermia. Biochim. Biophys. Acta Biomembr. 1993, 1149, 209–216. [Google Scholar] [CrossRef]
- Lu, T.; Ten Hagen, T.L. Inhomogeneous crystal grain formation in DPPC-DSPC based thermosensitive liposomes determines content release kinetics. J. Control. Release 2017, 247, 64–72. [Google Scholar] [CrossRef]
- Soares, D.C.F.; de Oliveira, M.C.; de Barros, A.L.B.; Cardoso, V.N.; Ramaldes, G.A. Liposomes radiolabeled with 159 Gd: In vitro antitumoral activity, biodistribution study and scintigraphic image in Ehrlich tumor bearing mice. Eur. J. Pharm. Sci. 2011, 43, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-J.; Liu, H.-W.; Yang, L.; Zhong, Y.-G.; Zheng, Y.-Z. Influence of membrane physical state on the lysosomal proton permeability. J. Membr. Biol. 2000, 175, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Kulig, W.; Tynkkynen, J.; Javanainen, M.; Manna, M.; Rog, T.; Vattulainen, I.; Jungwirth, P. How well does cholesteryl hemisuccinate mimic cholesterol in saturated phospholipid bilayers? J. Mol. Modeling 2014, 20, 2121. [Google Scholar] [CrossRef]
- Shabbits, J.A.; Mayer, L.D. Intracellular delivery of ceramide lipids via liposomes enhances apoptosis in vitro. Biochim. Biophys. Acta Biomembr. 2003, 1612, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Gubernator, J. Active methods of drug loading into liposomes: Recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin. Drug Deliv. 2011, 8, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New developments in liposomal drug delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef] [PubMed]
- Drazenovic, J.; Wang, H.; Roth, K.; Zhang, J.; Ahmed, S.; Chen, Y.; Bothun, G.; Wunder, S.L. Effect of lamellarity and size on calorimetric phase transitions in single component phosphatidylcholine vesicles. Biochim. Biophys. Acta Biomembr. 2015, 1848, 532–543. [Google Scholar] [CrossRef] [Green Version]
- Marsh, D.; Bartucci, R.; Sportelli, L. Lipid membranes with grafted polymers: Physicochemical aspects. Biochim. Biophys. Acta Biomembr. 2003, 1615, 33–59. [Google Scholar] [CrossRef] [Green Version]
- Neunert, G.; Tomaszewska-Gras, J.; Baj, A.; Gauza-Włodarczyk, M.; Witkowski, S.; Polewski, K. Phase Transitions and Structural Changes in DPPC Liposomes Induced by a 1-Carba-Alpha-Tocopherol Analogue. Molecules 2021, 26, 2851. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.; Park, J.-Y.; Wright, A.M.; Tong, J. Materials characterization of the low temperature sensitive liposome (LTSL): Effects of the lipid composition (lysolipid and DSPE–PEG2000) on the thermal transition and release of doxorubicin. Faraday Discuss. 2013, 161, 515–534. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar] [CrossRef]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2012, 64, 24–36. [Google Scholar] [CrossRef]
- Semple, S.C.; Chonn, A.; Cullis, P.R. Influence of cholesterol on the association of plasma proteins with liposomes. Biochemistry 1996, 35, 2521–2525. [Google Scholar] [CrossRef]
- Lee, H.; Larson, R.G. Adsorption of plasma proteins onto PEGylated lipid bilayers: The effect of PEG size and grafting density. Biomacromolecules 2016, 17, 1757–1765. [Google Scholar] [CrossRef] [Green Version]
- Crommelin, D.; Van Bommel, E. Stability of liposomes on storage: Freeze dried, frozen or as an aqueous dispersion. Pharm. Res. 1984, 1, 159–163. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef]
- O’Driscoll, K.; Sanayei, R.A. Chain-length dependence of the glass transition temperature. Macromolecules 1991, 24, 4479–4480. [Google Scholar] [CrossRef]
- Prislan, I.; Lokar, M.; Zirdum, M.; Valant, J.; Ulrih, N.P. Contribution of headgroup and chain length of glycerophospholipids to thermal stability and permeability of liposomes loaded with calcein. Chem. Phys. Lipids 2019, 225, 104807. [Google Scholar] [CrossRef]
- Grit, M.; Crommelin, D.J. Chemical stability of liposomes: Implications for their physical stability. Chem. Phys. Lipids 1993, 64, 3–18. [Google Scholar] [CrossRef]
- Alves, A.C.; Magarkar, A.; Horta, M.; Lima, J.L.; Bunker, A.; Nunes, C.; Reis, S. Influence of doxorubicin on model cell membrane properties: Insights from in vitro and in silico studies. Sci. Rep. 2017, 7, 6343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isom, D.G.; Castañeda, C.A.; Cannon, B.R. Large shifts in pKa values of lysine residues buried inside a protein. Proc. Natl. Acad. Sci. USA 2011, 108, 5260–5265. [Google Scholar] [CrossRef] [Green Version]
- Fitch, C.A.; Karp, D.A.; Lee, K.K.; Stites, W.E.; Lattman, E.E.; García-Moreno, E.B. Experimental pKa values of buried residues: Analysis with continuum methods and role of water penetration. Biophys. J. 2002, 82, 3289–3304. [Google Scholar] [CrossRef] [Green Version]
- Ellens, H.; Bentz, J.; Szoka, F.C. pH-induced destabilization of phosphatidylethanolamine-containing liposomes: Role of bilayer contact. Biochemistry 1984, 23, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, K.; Argyrakis, P.; Macheras, P. Fractal kinetics in drug release from finite fractal matrices. J. Chem. Phys. 2003, 119, 6373–6377. [Google Scholar] [CrossRef] [Green Version]







| Group | Lipid Composition | Molar Ratio | Type |
|---|---|---|---|
| A | DPPC | 1 | Non-long-circulating and thermosensitive |
| DPPC/CHEMS | 9:1 | Non-long-circulating, fusogenic, thermo- and pH-sensitive | |
| DPPC/CHEMS/DSPE-PEG | 80:15:5 | Long-circulating, fusogenic, thermo- and pH-sensitive | |
| B | DPPC/DSPC | 3:1 | Non-long-circulating and thermo-sensitive |
| DPPC/DSPC/CHEMS | 7:2:1 | Mid-long-circulating, fusogenic thermo- and pH-sensitive | |
| DPPC/DSPC/CHEMS/DSPE-PEG | 60:20:15:5 | Long-circulating, fusogenic, thermo- and pH-sensitive | |
| C | DPPC/DOPE | 7:3 | Non-long-circulating, fusogenic, pH-sensitive |
| DPPC/DOPE/CHEMS | 6:3:1 | Non-long-circulating, fusogenic, pH-sensitive | |
| DPPC/DOPE/CHEMS/DSPE-PEG | 50:30:15:5 | Long-circulating, fusogenic, pH-sensitive |
| Group | Lipid Composition | DH (nm) | PDI | EE% | Isoelectric pt. | Tm (°C) |
|---|---|---|---|---|---|---|
| A | DPPC | 116 ± 3 | 0.18 ± 0.02 | 96.9 ± 0.5 | 7.80 | 41.40 ± 0.02 |
| DPPC/CHEMS | 149 ± 7 | 0.21 ± 0.01 | 95 ± 1 | 5.61 | 38.2 ± 0.2 | |
| DPPC/CHEMS/DSPE-PEG | 156 ± 18 | 0.20 ± 0.02 | 94 ± 3 | 6.22 | 41.34 ± 0.02 | |
| B | DPPC/DSPC | 157 ± 1 | 0.22 ± 0.03 | 88 ± 2 | 5.95 | 42.4 ± 0.2 |
| DPPC/DSPC/CHEMS | 160 ± 1 | 0.20 ± 0.01 | 92 ± 2 | 5.37 | 39.7 ± 0.1 | |
| DPPC/DSPC/CHEMS/DSPE-PEG | 187 ± 4 | 0.22 ± 0.02 | 92 ± 5 | 5.61 | 41.4 ± 0.2 | |
| C | DPPC/DOPE | 111 ± 17 | 0.24 ± 0.07 | 96.7 ± 0.5 | 8.00 | * |
| DPPC/DOPE/CHEMS | 118 ± 4 | 0.20 ± 0.04 | 87 ± 12 | 5.49 | * | |
| DPPC/DOPE/CHEMS/DSPE-PEG | 117.39 ± 0.03 | 0.23 ± 0.06 | 93 ± 4 | 5.13 | * |
| Group | Lipid Composition | 42 °C, pH 5.5 | 42 °C, pH 7.4 | 37 °C, pH 5.5 | 37 °C, pH 7.4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| m | Ratio | m | Ratio | m | Ratio | m | Ratio | ||
| A | DPPC | 4.04 | 5.67 | 1.77 | 1.47 | 1.30 | 2.21 | 0.68 | 1 |
| DPPC/CHEMS | 6.39 | 1.91 | 2.58 | 1.12 | 5.92 | 1.25 | 2.70 | 1 | |
| DPPC/CHEMS/DSPE-PEG | 4.71 | 2.30 | 2.39 | 1.54 | 3.53 | 1.74 | 2.52 | 1 | |
| B | DPPC/DSPC | 6.39 | 1.86 | 2.32 | 0.59 | 5.89 | 1.12 | 2.7 | 1 |
| DPPC/DSPC/CHEMS | 4.23 | 2.88 | 1.77 | 1.51 | 5.25 | 2.92 | 0.98 | 1 | |
| DPPC/DSPC/CHEMS/DSPE-PEG | 7.21 | 1.75 | 1.74 | 0.93 | 6.19 | 1.71 | 2.11 | 1 | |
| C | DPPC/DOPE | 4.04 | 2.48 | 2.70 | 2.01 | 4.20 | 1.88 | 0.79 | 1 |
| DPPC/DOPE/CHEMS | 5.83 | 2.42 | 3.26 | 1.48 | 3.15 | 1.37 | 2.67 | 1 | |
| DPPC/DOPE/CHEMS/DSPE-PEG | 3.42 | 1.66 | 1.69 | 0.93 | 1.83 | 1.82 | 1.03 | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, B.D.; Cardoso, V.F.; Lanceros-Méndez, S.; Castanheira, E.M.S. Solid Magnetoliposomes as Multi-Stimuli-Responsive Systems for Controlled Release of Doxorubicin: Assessment of Lipid Formulations. Biomedicines 2022, 10, 1207. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10051207
Cardoso BD, Cardoso VF, Lanceros-Méndez S, Castanheira EMS. Solid Magnetoliposomes as Multi-Stimuli-Responsive Systems for Controlled Release of Doxorubicin: Assessment of Lipid Formulations. Biomedicines. 2022; 10(5):1207. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10051207
Chicago/Turabian StyleCardoso, Beatriz D., Vanessa F. Cardoso, Senetxu Lanceros-Méndez, and Elisabete M. S. Castanheira. 2022. "Solid Magnetoliposomes as Multi-Stimuli-Responsive Systems for Controlled Release of Doxorubicin: Assessment of Lipid Formulations" Biomedicines 10, no. 5: 1207. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10051207