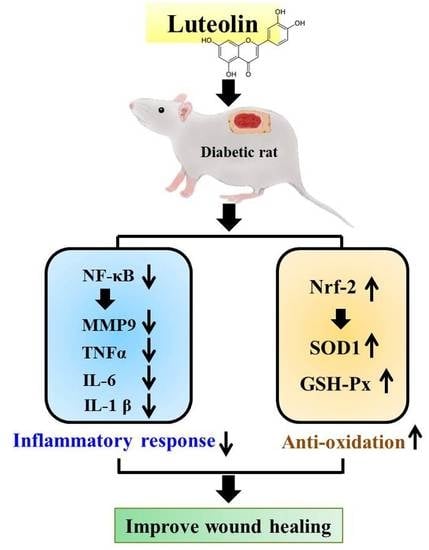
Therapeutic Potential of Luteolin on Impaired Wound Healing in Streptozotocin-Induced Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Wound Induction
2.3. Histological Analysis
2.4. Immunohistochemical Staining
2.5. Western Blot Analysis
2.6. Immunofluorescence
2.7. Statistical Analysis
3. Results
3.1. Luteolin Attenuates Blood Glucose Level in STZ-Induced Diabetic Rats
3.2. Luteolin Accelerates Wound Re-epithelization and Inflammatory Response in STZ-Induced Diabetic Rats
3.3. Luteolin Attenuates NF-κB-Induced Inflammatory Response and Nrf2-Mediated Oxidative Response in STZ-Induced Diabetic Rats
3.4. Luteolin Accelerates Angiogenesis and Neuronal Regeneration in STZ-Induced Diabetic Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| STZ | streptozotocin |
| MMP-9 | matrix metalloproteinase-9 |
| TNF-α | tumor necrosis factor-α |
| IL-6 | interleukin-6 |
| SOD1 | superoxide dismutase |
| GSH-Px | glutathione peroxidase |
| Nrf-2 | nuclear factor erythroid 2-related factor |
| VEGF | vascular endothelial growth factor |
| UCH-L1 | ubiquitin carboxy-terminal hydrolase-1 |
References
- Chen, L.Y.; Huang, C.N.; Liao, C.K.; Chang, H.M.; Kuan, Y.H.; Tseng, T.J.; Yen, K.J.; Yang, K.L.; Lin, H.C. Effects of Rutin on Wound Healing in Hyperglycemic Rats. Antioxidants 2020, 9, 1122. [Google Scholar] [CrossRef]
- Donnelly, R.; Emslie-Smith, A.M.; Gardner, I.D.; Morris, A.D. ABC of arterial and venous disease: Vascular complications of diabetes. BMJ 2000, 320, 1062–1066. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Jhamb, S.; Vangaveti, V.N.; Malabu, U.H. Genetic and molecular basis of diabetic foot ulcers: Clinical review. J. Tissue Viability 2016, 25, 229–236. [Google Scholar] [CrossRef]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70. [Google Scholar] [CrossRef]
- Wu, X.; Yang, L.; Zheng, Z.; Li, Z.; Shi, J.; Li, Y.; Han, S.; Gao, J.; Tang, C.; Su, L.; et al. Src promotes cutaneous wound healing by regulating MMP-2 through the ERK pathway. Int. J. Mol. Med. 2016, 37, 639–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farghali, H.A.; AbdElKader, N.A.; Khattab, M.S.; AbuBakr, H.O. Evaluation of subcutaneous infiltration of autologous platelet-rich plasma on skin-wound healing in dogs. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tombulturk, F.K.; Soydas, T.; Sarac, E.Y.; Tuncdemir, M.; Coskunpinar, E.; Polat, E.; Sirekbasan, S.; Kanigur-Sultuybek, G. Regulation of MMP 2 and MMP 9 expressions modulated by AP-1 (c-jun) in wound healing: Improving role of Lucilia sericata in diabetic rats. Acta Diabetol. 2019, 56, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, U.A.; DiPietro, L.A. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemnia, M.; Nikousefat, Z.; Mohammadalipour, A.; Zangeneh, M.M.; Zangeneh, A. Wound healing activity of Pimpinella anisum methanolic extract in streptozotocin-induced diabetic rats. J. Wound Care 2019, 28, S26–S36. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Cheng, H.L.; Lin, L.Y.; Chen, S.C.; Hsu, C.L. The potential role of phenolic compounds on modulating gut microbiota in obesity. J. Food Drug Anal. 2020, 28. [Google Scholar] [CrossRef]
- Ren, B.; Qin, W.; Wu, F.; Wang, S.; Pan, C.; Wang, L.; Zeng, B.; Ma, S.; Liang, J. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur. J. Pharmacol. 2016, 773, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Chen, P.J.; Chang, S.H.; Weng, Y.T.; Chang, F.R.; Chang, K.Y.; Chen, C.Y.; Kao, T.I.; Hwang, T.L. Luteolin attenuates neutrophilic oxidative stress and inflammatory arthritis by inhibiting Raf1 activity. Biochem. Pharmacol. 2018, 154, 384–396. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Tome, S.M.; Silva, A.M.; Porto, G.; Fernandes, E. Modulation of human neutrophils’ oxidative burst by flavonoids. Eur. J. Med. Chem. 2013, 67, 280–292. [Google Scholar] [CrossRef]
- Ambasta, R.K.; Gupta, R.; Kumar, D.; Bhattacharya, S.; Sarkar, A.; Kumar, P. Can luteolin be a therapeutic molecule for both colon cancer and diabetes? Brief. Funct. Genom. 2018, 18, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Wozniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xagorari, A.; Papapetropoulos, A.; Mauromatis, A.; Economou, M.; Fotsis, T.; Roussos, C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J. Pharmacol. Exp. Ther. 2001, 296, 181–187. [Google Scholar]
- Ferrari, C.K.; Torres, E.A. Biochemical pharmacology of functional foods and prevention of chronic diseases of aging. Biomed. Pharmacother. 2003, 57, 251–260. [Google Scholar] [CrossRef]
- Lim, D.Y.; Cho, H.J.; Kim, J.; Nho, C.W.; Lee, K.W.; Park, J.H. Luteolin decreases IGF-II production and downregulates insulin-like growth factor-I receptor signaling in HT-29 human colon cancer cells. BMC Gastroenterol. 2012, 12, 9. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Hyun, Y.J.; Park, J.E.; Shilnikova, K.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Jeong, Y.J.; et al. Luteolin induces apoptotic cell death via antioxidant activity in human colon cancer cells. Int. J. Oncol. 2017, 51, 1169–1178. [Google Scholar] [CrossRef] [Green Version]
- El-Bassossy, H.M.; Abo-Warda, S.M.; Fahmy, A. Chrysin and luteolin attenuate diabetes-induced impairment in endothelial-dependent relaxation: Effect on lipid profile, AGEs and NO generation. Phytother. Res. 2013, 27, 1678–1684. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.X.; Cheng, X.J.; Luo, X.; Yang, X.; Pang, Y.P.; Zhang, X.F.; Zhang, Y.Y.; Liu, Y. Luteolin Ameliorates Cognitive Impairments by Suppressing the Expression of Inflammatory Cytokines and Enhancing Synapse-Associated Proteins GAP-43 and SYN Levels in Streptozotocin-Induced Diabetic Rats. Neurochem. Res. 2018, 43, 1905–1913. [Google Scholar] [CrossRef]
- Deqiu, Z.; Kang, L.; Jiali, Y.; Baolin, L.; Gaolin, L. Luteolin inhibits inflammatory response and improves insulin sensitivity in the endothelium. Biochimie 2011, 93, 506–512. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, W.; Yun, J.M. Luteolin inhibits hyperglycemia-induced proinflammatory cytokine production and its epigenetic mechanism in human monocytes. Phytother. Res. 2014, 28, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Gerharz, M.; Baranowsky, A.; Siebolts, U.; Eming, S.; Nischt, R.; Krieg, T.; Wickenhauser, C. Morphometric analysis of murine skin wound healing: Standardization of experimental procedures and impact of an advanced multitissue array technique. Wound Repair Regen. 2007, 15, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Birt, D.F.; Hendrich, S.; Wang, W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol. Ther. 2001, 90, 157–177. [Google Scholar] [CrossRef]
- Moiseev Iu, B.; Korzhen’iants, V.A.; Biriukov, A.A. Effects of preliminary loading on dynamic strength of the spine. Aviakosm Ekol. Med. 1996, 30, 31–34. [Google Scholar]
- Casimir, M.; Chaffard, G.; Maechler, P. Resveratrol long-term treatment differentiates INS-1E beta-cell towards improved glucose response and insulin secretion. Pflugers Arch. 2019, 471, 337–345. [Google Scholar] [CrossRef]
- Rouse, M.; Younes, A.; Egan, J.M. Resveratrol and curcumin enhance pancreatic beta-cell function by inhibiting phosphodiesterase activity. J. Endocrinol. 2014, 223, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; He, S.; Shen, Y.; Zhang, J.; Wan, Y.; Tang, X.; Liu, S.; Yao, X. Natural Product Luteolin Rescues THAP-Induced Pancreatic beta-Cell Dysfunction through HNF4alpha Pathway. Am. J. Chin. Med. 2020, 48, 1435–1454. [Google Scholar] [CrossRef]
- Lodhi, S.; Singhai, A.K. Wound healing effect of flavonoid rich fraction and luteolin isolated from Martynia annua Linn. on streptozotocin induced diabetic rats. Asian Pac. J. Trop Med. 2013, 6, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Jangde, R.; Srivastava, S.; Singh, M.R.; Singh, D. In vitro and In vivo characterization of quercetin loaded multiphase hydrogel for wound healing application. Int. J. Biol. Macromol. 2018, 115, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Ponrasu, T.; Jamuna, S.; Mathew, A.; Madhukumar, K.N.; Ganeshkumar, M.; Iyappan, K.; Suguna, L. Efficacy of L-proline administration on the early responses during cutaneous wound healing in rats. Amino Acids 2013, 45, 179–189. [Google Scholar] [CrossRef]
- Fikru, A.; Makonnen, E.; Eguale, T.; Debella, A.; Abie Mekonnen, G. Evaluation of in vivo wound healing activity of methanol extract of Achyranthes aspera L. J. Ethnopharmacol. 2012, 143, 469–474. [Google Scholar] [CrossRef]
- Barnes, P.J.; Karin, M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latha, R.C.; Daisy, P. Insulin-secretagogue, antihyperlipidemic and other protective effects of gallic acid isolated from Terminalia bellerica Roxb. in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2011, 189, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, W.; Qian, Y.; Zhu, W.; Qian, J.; Li, J.; Jin, Y.; Xu, X.; Liang, G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-kappaB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine 2019, 59, 152774. [Google Scholar] [CrossRef]
- Tan, X.; Yang, Y.; Xu, J.; Zhang, P.; Deng, R.; Mao, Y.; He, J.; Chen, Y.; Zhang, Y.; Ding, J.; et al. Luteolin Exerts Neuroprotection via Modulation of the p62/Keap1/Nrf2 Pathway in Intracerebral Hemorrhage. Front. Pharmacol. 2019, 10, 1551. [Google Scholar] [CrossRef]
- Andres, C.; Hasenauer, J.; Ahn, H.S.; Joseph, E.K.; Isensee, J.; Theis, F.J.; Allgower, F.; Levine, J.D.; Dib-Hajj, S.D.; Waxman, S.G.; et al. Wound-healing growth factor, basic FGF, induces Erk1/2-dependent mechanical hyperalgesia. Pain 2013, 154, 2216–2226. [Google Scholar] [CrossRef]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Nicol, G.D.; Vasko, M.R. Unraveling the story of NGF-mediated sensitization of nociceptive sensory neurons: ON or OFF the Trks? Mol. Interv. 2007, 7, 26–41. [Google Scholar] [CrossRef]
- Kabuta, T.; Wada, K. Insights into links between familial and sporadic Parkinson’s disease: Physical relationship between UCH-L1 variants and chaperone-mediated autophagy. Autophagy 2008, 4, 827–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Sugiura, Y.; Myers, K.G.; Liu, Y.; Lin, W. Ubiquitin carboxyl-terminal hydrolase L1 is required for maintaining the structure and function of the neuromuscular junction. Proc. Natl. Acad. Sci. USA 2010, 107, 1636–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Fallon, L.; Lashuel, H.A.; Liu, Z.; Lansbury, P.T., Jr. The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson’s disease susceptibility. Cell 2002, 111, 209–218. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-Y.; Cheng, H.-L.; Kuan, Y.-H.; Liang, T.-J.; Chao, Y.-Y.; Lin, H.-C. Therapeutic Potential of Luteolin on Impaired Wound Healing in Streptozotocin-Induced Rats. Biomedicines 2021, 9, 761. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9070761
Chen L-Y, Cheng H-L, Kuan Y-H, Liang T-J, Chao Y-Y, Lin H-C. Therapeutic Potential of Luteolin on Impaired Wound Healing in Streptozotocin-Induced Rats. Biomedicines. 2021; 9(7):761. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9070761
Chicago/Turabian StyleChen, Li-You, Hsin-Lin Cheng, Yu-Hsiang Kuan, Tang-Jun Liang, Yun-Yi Chao, and Hsing-Chun Lin. 2021. "Therapeutic Potential of Luteolin on Impaired Wound Healing in Streptozotocin-Induced Rats" Biomedicines 9, no. 7: 761. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9070761