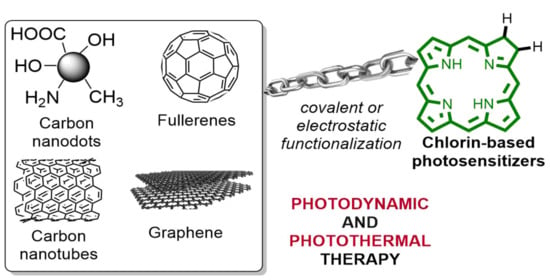
Learning from Nature: Bioinspired Chlorin-Based Photosensitizers Immobilized on Carbon Materials for Combined Photodynamic and Photothermal Therapy
Abstract
:1. Introduction
2. Photodynamic and Photothermal Therapy: Mechanism and Applications
2.1. Mechanisms of Photodynamic and Photothermal Therapy
2.2. Anticancer and Antimicrobial Applications of PDT
3. Bioinspired Photosensitizers
4. Carbon Materials Applied in Photodynamic/Photothermal Therapy
5. An Update on Chlorin-Based Photosensitizers Immobilized on Carbon Materials for Photodynamic and Photothermal Therapy
5.1. Carbon Dots
5.2. Fullerene
5.3. Carbon Nanotubes
5.4. Graphene
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.J.; Chen, T.F. Transition metal complexes as photosensitizers for integrated cancer theranostic applications. Coord. Chem. Rev. 2020, 418, 20. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Choi, G.; Choy, J.H. Recent trends in nano photo-chemo therapy approaches and future scopes. Coord. Chem. Rev. 2020, 411, 23. [Google Scholar] [CrossRef]
- Vinagreiro, C.S.; Zangirolami, A.; Schaberle, F.A.; Nunes, S.C.C.; Blanco, K.C.; Inada, N.M.; da Silva, G.J.; Pais, A.; Bagnato, V.S.; Arnaut, L.G.; et al. Antibacterial Photodynamic Inactivation of Antibiotic-Resistant Bacteria and Biofilms with Nanomolar Photosensitizer Concentrations. ACS Infect. Dis. 2020, 6, 1517–1526. [Google Scholar] [CrossRef]
- Dias, L.D.; Bagnato, V.S. An update on clinical photodynamic therapy for fighting respiratory tract infections: A promising tool against COVID-19 and its co-infections. Laser Phys. Lett. 2020, 17, 9. [Google Scholar] [CrossRef]
- Jia, Q.Y.; Song, Q.; Li, P.; Huang, W. Rejuvenated Photodynamic Therapy for Bacterial Infections. Adv. Healthc. Mater. 2019, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Q.; Jiang, X.F.; Fan, T.J.; Zheng, Z.W.; Liu, Z.Y.; Chen, Y.B.; Cao, L.Q.; Xie, Z.J.; Zhang, D.W.; Zhao, J.Q.; et al. Recent advances in photodynamic therapy based on emerging two-dimensional layered nanomaterials. Nano Res. 2020, 13, 1485–1508. [Google Scholar] [CrossRef]
- Bayona, A.M.D.; Mroz, P.; Thunshelle, C.; Hamblin, M.R. Design features for optimization of tetrapyrrole macrocycles as antimicrobial and anticancer photosensitizers. Chem. Biol. Drug Des. 2017, 89, 192–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucelik, B.; Sulek, A.; Dabrowski, J.M.D. Bacteriochlorins and their metal complexes as NIR-absorbing photosensitizers: Properties, mechanisms, and applications. Coord. Chem. Rev. 2020, 416, 47. [Google Scholar] [CrossRef]
- Sayyad-Amin, P.; Jahansooz, M.R.; Borzouei, A.; Ajili, F. Changes in photosynthetic pigments and chlorophyll-a fluorescence attributes of sweet-forage and grain sorghum cultivars under salt stress. J. Biol. Phys. 2016, 42, 601–620. [Google Scholar] [CrossRef] [Green Version]
- Sivasankarapillai, V.S.; Kirthi, A.V.; Akksadha, M.; Indu, S.; Dharshini, U.D.; Pushpamalar, J.; Karthik, L. Recent advancements in the applications of carbon nanodots: Exploring the rising star of nanotechnology. Nanoscale Adv. 2020, 2, 1760–1773. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.; Tao, R.; Wang, Z. Carbon-based materials for photodynamic therapy: A mini-review. Front. Chem. Sci. Eng. 2019, 13, 310–323. [Google Scholar] [CrossRef]
- Xu, Y.H.; Shan, Y.L.; Cong, H.L.; Shen, Y.Q.; Yu, B. Advanced Carbon-based Nanoplatforms Combining Drug Delivery and Thermal Therapy for Cancer Treatment. Curr. Pharm. Des. 2018, 24, 4060–4076. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, N.M. 30 years of advances in functionalization of carbon nanomaterials for biomedical applications: A practical review. J. Mater. Res. 2017, 32, 107–127. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhang, X.B.; Yang, R.H.; Zhu, Z.; Chen, Y.; Tan, W.H. Single-walled carbon nanotubes as optical materials for biosensing. Nanoscale 2011, 3, 1949–1956. [Google Scholar] [CrossRef]
- Andersen, A.J.; Wibroe, P.P.; Moghimi, S.M. Perspectives on carbon nanotube-mediated adverse immune effects. Adv. Drug Deliv. Rev. 2012, 64, 1700–1705. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.T.; Wu, F.; Zheng, T.; Ashley, J.; Mohammadniaei, M.; Zhang, Q.C.; Wang, M.Q.; Li, L.; Shen, J.; et al. Biodegradable Poly(gamma-glutamic acid)@glucose oxidase@carbon dot nanoparticles for simultaneous multimodal imaging and synergetic cancer therapy. Biomaterials 2020, 252, 12. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, D.M.; Liu, Y.; Jiang, B.; Jiang, W.; Yan, X.Y.; Fan, K.L. Platinum-carbon-integrated nanozymes for enhanced tumor photodynamic and photothermal therapy. Nanoscale 2020, 12, 13548–13557. [Google Scholar] [CrossRef]
- Zhao, S.J.; Wu, S.L.; Jia, Q.Y.; Huang, L.; Lan, M.H.; Wang, P.F.; Zhang, W.J. Lysosome-targetable carbon dots for highly efficient photothermal/photodynamic synergistic cancer therapy and photoacoustic/two-photon excited fluorescence imaging. Chem. Eng. J. 2020, 388, 9. [Google Scholar] [CrossRef]
- Zhang, F.R.; Liu, Y.H.; Lei, J.N.; Wang, S.H.; Ji, X.M.; Liu, H.Y.; Yang, Q. Metal-Organic-Framework-Derived Carbon Nanostructures for Site-Specific Dual-Modality Photothermal/Photodynamic Thrombus Therapy. Adv. Sci. 2019, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Moan, J.; Peng, Q. An outline of the hundred-year history of PDT. Anticancer Res. 2003, 23, 3591–3600. [Google Scholar]
- Tampa, M.; Sarbu, M.I.; Matei, C.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Constantin, C.; Neagu, M.; Georgescu, S.R. Photodynamic therapy: A hot topic in dermato-oncology. Oncol. Lett. 2019, 17, 4085–4093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diogo, P.; Gonalves, T.; Palma, P.; Santos, J.M. Photodynamic Antimicrobial Chemotherapy for Root Canal System Asepsis: A Narrative Literature Review. Int. J. Dent. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moan, J.; Berg, K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.A.; Evans, D.H.; Abraharnse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B-Biol. 2009, 96, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Plaetzer, K.; Berneburg, M.; Kiesslich, T.; Maisch, T. New Applications of Photodynamic Therapy in Biomedicine and Biotechnology. Biomed Res. Int. 2013, 2013, 3. [Google Scholar] [CrossRef]
- Hanakova, A.; Bogdanova, K.; Tomankova, K.; Pizova, K.; Malohlava, J.; Binder, S.; Bajgar, R.; Langova, K.; Kolar, M.; Mosinger, J.; et al. The application of antimicrobial photodynamic therapy on S. aureus and E. coli using porphyrin photosensitizers bound to cyclodextrin. Microbiol. Res. 2014, 169, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Doughty, A.C.V.; Hoover, A.R.; Layton, E.; Murray, C.K.; Howard, E.W.; Chen, W.R. Nanomaterial Applications in Photothermal Therapy for Cancer. Materials 2019, 12, 779. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.H.; Li, Z.B.; Sun, Z.B.; Shao, J.D.; Yu, X.F.; Guo, Z.N.; Wang, J.H.; Xiao, Q.L.; Wang, H.Y.; Wang, Q.Q.; et al. Metabolizable Ultrathin Bi2Se3 Nanosheets in Imaging-Guided Photothermal Therapy. Small 2016, 12, 4136–4145. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Melamed, J.R.; Edelstein, R.S.; Day, E.S. Elucidating the Fundamental Mechanisms of Cell Death Triggered by Photothermal Therapy. ACS Nano 2015, 9, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Yang, W.X.; Guan, Z.Z.; Yu, W.F.; Fan, B.; Xu, N.Z.; Liao, D.J. There are only four basic modes of cell death, although there are many ad-hoc variants adapted to different situations. Cell Biosci. 2018, 8, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugan, C.; Sharma, V.; Murugan, R.K.; Malaimegu, G.; Sundaramurthy, A. Two-dimensional cancer theranostic nanomaterials: Synthesis, surface functionalization and applications in photothermal therapy. J. Control. Release 2019, 299, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, C.; Cui, W.; Gong, H.; Liang, C.; Shi, X.Z.; Li, Z.W.; Sun, B.Q.; Liu, Z. Combined photothermal and photodynamic therapy delivered by PEGylated MoS2 nanosheets. Nanoscale 2014, 6, 11219–11225. [Google Scholar] [CrossRef]
- Gao, D.; Guo, X.; Zhang, X.; Chen, S.; Wang, Y.; Chen, T.; Huang, G.; Gao, Y.; Tian, Z.; Yang, Z. Multifunctional phototheranostic nanomedicine for cancer imaging and treatment. Mater. Today Bio 2020, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Dahle, J.; Kaalhus, O.; Moan, J.; Steen, H.B. Cooperative effects of photodynamic treatment of cells in microcolonies. Proc. Natl. Acad. Sci. USA 1997, 94, 1773–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abels, C. Targeting of the vascular system of solid tumours by photodynamic therapy (PDT). Photochem. Photobiol. Sci. 2004, 3, 765–771. [Google Scholar] [CrossRef]
- Chen, C.Y.; Liu, J.; Wang, J.; Wu, X.C.; Gu, Z.J. Near-infrared light-mediated nanomaterials as a precision nanomedicine for in vivo multimodal imaging-guided cancer thermo-chemotherapy. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 455. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Sun, Z.R.; Ren, Y.; Chen, X.; Zhang, W.; Zhu, X.H.; Mao, Z.W.; Shen, J.L.; Nie, S.N. Advances in nanomaterials for use in photothermal and photodynamic therapeutics. Mol. Med. Rep. 2019, 20, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Pucelik, B.; Sulek, A.; Drozd, A.; Stochel, G.; Pereira, M.M.; Pinto, S.M.A.; Arnaut, L.G.; Dabrowski, J.M. Enhanced Cellular Uptake and Photodynamic Effect with Amphiphilic Fluorinated Porphyrins: The Role of Sulfoester Groups and the Nature of Reactive Oxygen Species. Int. J. Mol. Sci. 2020, 21, 2786. [Google Scholar] [CrossRef] [Green Version]
- Mesquita, M.Q.; Dias, C.J.; Gamelas, S.; Fardilha, M.; Neves, M.; Faustino, M.A.F. An Insight on the role of photosensitizer nanocarriers for Photodynamic Therapy. An. Acad. Bras. Cienc. 2018, 90, 1101–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahimova, V.; Denisov, S.A.; Vanvarenberg, K.; Verwilst, P.; Preat, V.; Guigner, J.M.; McClenaghan, N.D.; Lecommandoux, S.; Fustin, C.A. Photosensitizer localization in amphiphilic block copolymers controls photodynamic therapy efficacy. Nanoscale 2017, 9, 11180–11186. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, J.; Luo, L.H.; Jiang, M.S.; Qin, B.; Yin, H.; Zhu, C.Q.; Yuan, X.L.; Zhang, J.L.; Luo, Z.Y.; et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat. Commun. 2019, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Hoorelbeke, D.; Decrock, E.; Van Haver, V.; De Bock, M.; Leybaert, L. Calcium, a pivotal player in photodynamic therapy? Biochim. Biophys. Acta-Mol. Cell Res. 2018, 1865, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.Y.; Chiu, S.M.; Oleinick, N.L. Photodynamic therapy-induced death of MCF-7 human breast cancer cells: A role for caspase-3 in the late steps of apoptosis but not for the critical lethal event. Exp. Cell Res. 2001, 263, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Z.; Li, J.Y.; Su, Y.Q.; Yang, L.Q.; Chen, L.; Qiang, L.; Wang, Y.J.; Xiang, H.J.; Tham, H.P.; Peng, J.J.; et al. MTH1 inhibitor amplifies the lethality of reactive oxygen species to tumor in photodynamic therapy. Sci. Adv. 2020, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.H.; Yin, G.F.; Le, V.; Zhang, A.L.; Chen, S.Y.; Liang, X.; Liu, J.W. Photodynamic-therapy Activates Immune Response by disrupting Immunity Homeostasis of Tumor Cells, which Generates Vaccine for Cancer Therapy. Int. J. Biol. Sci. 2016, 12, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Fernald, K.; Kurokawa, M. Evading apoptosis in cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 14. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, H.; Bahador, A.; Pourhajibagher, M.; Alikhani, M.Y. Antimicrobial Photodynamic Therapy: An Effective Alternative Approach to Control Bacterial Infections. J. Lasers Med. Sci. 2018, 9, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Diogo, P.; Faustino, M.A.F.; Neves, M.; Palma, P.J.; Baptista, I.P.; Goncalves, T.; Santos, J.M. An Insight into Advanced Approaches for Photosensitizer Optimization in Endodontics-A Critical Review. J. Funct. Biomater. 2019, 10, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diogo, P.; Mota, M.; Fernandes, C.; Sequeira, D.; Palma, P.; Caramelo, F.; Neves, M.; Faustino, M.A.F.; Goncalves, T.; Santos, J.M. Is the chlorophyll derivative Zn(II)e(6)Me a good photosensitizer to be used in root canal disinfection? Photodiagnosis Photodyn. Ther. 2018, 22, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Deng, D.M.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.Z.; He, J.; Yu, W.M.; Li, Y.C.; Liu, Z.H.; Zhou, B.N.; Liu, Y.M. A promising anticancer drug: A photosensitizer based on the porphyrin skeleton. RSC Med. Chem. 2020, 11, 427–437. [Google Scholar] [CrossRef]
- Filip, A.G.; Clichici, S.; Daicoviciu, D.; Ion, R.M.; Tatomir, C.; Rogojan, L.; Opris, I.; Mocan, T.; Olteanu, D.; Muresan, A. Possible in vivo mechanisms involved in photodynamic therapy using tetrapyrrolic macrocycles. Braz. J. Med. Biol. Res. 2011, 44, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, R.C.E.; da Silva, L.O.; Bartolomeu, A.D.; Brocksom, T.J.; de Oliveira, K.T. Recent applications of porphyrins as photocatalysts in organic synthesis: Batch and continuous flow approaches. Beilstein J. Org. Chem. 2020, 16, 917–955. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.M.; Dias, L.D.; Calvete, M.J.F. Metalloporphyrins: Bioinspired Oxidation Catalysts. ACS Catal. 2018, 8, 10784–10808. [Google Scholar] [CrossRef]
- Li, J.; Li, T.; Duan, Y.R.; Li, H. “Even” conducting superiority in molecular wires designed by porphyrin and graphene nanoribbons. Mater. Des. 2020, 189, 9. [Google Scholar] [CrossRef]
- Bressan, G.; Cammidge, A.N.; Jones, G.A.; Heisler, I.A.; Gonzalez-Lucas, D.; Remiro-Buenamanana, S.; Meech, S.R. Electronic Energy Transfer in a Subphthalocyanine-Zn Porphyrin Dimer Studied by Linear and Nonlinear Ultrafast Spectroscopy. J. Phys. Chem. A 2019, 123, 5724–5733. [Google Scholar] [CrossRef]
- Chen, J.X.; Jiang, S.B.; Wang, J.; Renukuntla, J.; Sirimulla, S.; Chen, J.J. A comprehensive review of cytochrome P450 2E1 for xenobiotic metabolism. Drug Metab. Rev. 2019, 51, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Sitte, E.; Senge, M.O. The Red Color of Life Transformed—Synthetic Advances and Emerging Applications of Protoporphyrin IX in Chemical Biology. Eur. J. Org. Chem. 2020, 2020, 3171–3191. [Google Scholar] [CrossRef] [PubMed]
- Battersby, A.R. Tetrapyrroles: The pigments of life. Nat. Prod. Rep. 2000, 17, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, J.M.; Arnaut, L.G. Photodynamic therapy (PDT) of cancer: From local to systemic treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef]
- Gomes-da-Silva, L.C.; Zhao, L.W.; Bezu, L.; Zhou, H.; Sauvat, A.; Liu, P.; Durand, S.; Leduc, M.; Souquere, S.; Loos, F.; et al. Photodynamic therapy with redaporfin targets the endoplasmic reticulum and Golgi apparatus. EMBO J. 2018, 37, 18. [Google Scholar] [CrossRef]
- Pereira, M.M.; Monteiro, C.J.P.; Simoes, A.V.C.; Pinto, S.M.A.; Arnaut, L.G.; Sa, G.F.F.; Silva, E.F.F.; Rocha, L.B.; Simoes, S.; Formosinho, S.J. Synthesis and photophysical properties of amphiphilic halogenated bacteriochlorins: New opportunities for photodynamic therapy of cancer. J. Porphyr. Phthalocyanines 2009, 13, 567–573. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S. Synthetic Chlorins, Possible Surrogates for Chlorophylls, Prepared by Derivatization of Porphyrins. Chem. Rev. 2017, 117, 344–535. [Google Scholar] [CrossRef]
- Uliana, M.P.; Pires, L.; Pratavieira, S.; Brocksom, T.J.; de Oliveira, K.T.; Bagnato, V.S.; Kurachi, C. Photobiological characteristics of chlorophyll a derivatives as microbial PDT agents. Photochem. Photobiol. Sci. 2014, 13, 1137–1145. [Google Scholar] [CrossRef]
- Pereira, M.M.; Abreu, A.R.; Goncalves, N.P.F.; Calvete, M.J.F.; Simoes, A.V.C.; Monteiro, C.J.P.; Arnaut, L.G.; Eusebio, M.E.; Canotilho, J. An insight into solvent-free diimide porphyrin reduction: A versatile approach for meso-aryl hydroporphyrin synthesis. Green Chem. 2012, 14, 1666–1672. [Google Scholar] [CrossRef]
- Silva, G.A. Nanotechnology approaches to crossing the blood-brain barrier and drug delivery to the CNS. BMC Neurosci. 2008, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Rauti, R.; Musto, M.; Bosi, S.; Prato, M.; Ballerini, L. Properties and behavior of carbon nanomaterials when interfacing neuronal cells: How far have we come? Carbon 2019, 143, 430–446. [Google Scholar] [CrossRef]
- Li, Q.; Song, J.; Besenbacher, F.; Dong, M.D. Two-Dimensional Material Confined Water. Acc. Chem. Res. 2015, 48, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.P.; Zhou, B.; Lin, Z.X.; Liang, H.; Shen, X.C. Recent Advances in Carbon Nanomaterials for Cancer Phototherapy. Chem. Eur. J. 2019, 25, 3993–4004. [Google Scholar] [CrossRef]
- Bacakova, L.; Pajorova, J.; Tomkova, M.; Matejka, R.; Broz, A.; Stepanovska, J.; Prazak, S.; Skogberg, A.; Siljander, S.; Kallio, P. Applications of Nanocellulose/Nanocarbon Composites: Focus on Biotechnology and Medicine. Nanomaterials 2020, 10, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwag, D.S.; Oh, N.M.; Oh, Y.T.; Oh, K.T.; Youn, Y.S.; Lee, E.S. Photodynamic therapy using glycol chitosan grafted fullerenes. Int. J. Pharm. 2012, 431, 204–209. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Szyniszewski, A.M.; Wahr, D.; Herrmann, H.C.; Simon, D.I.; Rogers, C.; Kramer, P.; Shear, W.; Yeung, A.C.; Shunk, K.A.; et al. Phase I drug and light dose-escalation trial of motexafin lutetium and far red light activation (phototherapy) in subjects with coronary artery disease undergoing percutaneous coronary intervention and stent deployment—Procedural and long-term results. Circulation 2003, 108, 1310–1315. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Zhang, C.H.; Huang, Y.D.; Sun, S.F.; Guan, W.C.; Yao, Y.H. Photodynamic anticancer activities of water-soluble C-60 derivatives and their biological consequences in a He La cell line. Chem. Biol. Interact. 2012, 195, 86–94. [Google Scholar] [CrossRef]
- Mroz, P.; Pawlak, A.; Satti, M.; Lee, H.; Wharton, T.; Gali, H.; Sarna, T.; Hamblin, M.R. Functionalized fullerenes mediate photodynamic killing of cancer cells: Type I versus Type II photochemical mechanism. Free Radic. Biol. Med. 2007, 43, 711–719. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.K.; Chiang, L.Y.; Hamblin, M.R. Photodynamic therapy with fullerenes in vivo: Reality or a dream? Nanomedicine 2011, 6, 1813–1825. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.Y.; Ma, L.J.; Liu, Y.; Chen, C.Y. Applications of Functionalized Fullerenes in Tumor Theranostics. Theranostics 2012, 2, 238–250. [Google Scholar] [CrossRef] [Green Version]
- Chin, K.K.; Chuang, S.C.; Hernandez, B.; Campos, L.M.; Selke, M.; Foote, C.S.; Garcia-Garibay, M.A. Photophysical properties of non-homoconjugated 1,2-dihydro, 1,2,3,4-tetrahydro and 1,2,3,4,5,6-hexahydro-C-60 derivatives. Photochem. Photobiol. Sci. 2008, 7, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Yamakoshi, Y. A highly water-soluble C-60-NVP copolymer: A potential material for photodynamic therapy. Chem. Commun. 2006, 4805–4807. [Google Scholar] [CrossRef] [PubMed]
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiti, D.; Tong, X.M.; Mou, X.Z.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front. Pharmacol. 2019, 9, 16. [Google Scholar] [CrossRef]
- Molaei, M.J. Carbon quantum dots and their biomedical and therapeutic applications: A review. RSC Adv. 2019, 9, 6460–6481. [Google Scholar] [CrossRef]
- Gulzar, A.; Xu, J.T.; Yang, D.; Xu, L.G.; He, F.; Gai, S.L.; Yang, P.P. Nano-graphene oxide-UCNP-Ce6 covalently constructed nanocomposites for NIR-mediated bioimaging and PTT/PDT combinatorial therapy. Dalton Trans. 2018, 47, 3931–3939. [Google Scholar] [CrossRef]
- McManus, D.; Vranic, S.; Withers, F.; Sanchez-Romaguera, V.; Macucci, M.; Yang, H.F.; Sorrentino, R.; Parvez, K.; Son, S.K.; Iannaccone, G.; et al. Water-based and biocompatible 2D crystal inks for all-inkjet-printed heterostructures. Nat. Nanotechnol. 2017, 12, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Bleeker, E.A.; Brand, W.; Cassee, F.R.; van Elk, M.; Gosens, I.; de Jong, W.H.; Meesters, J.A.J.; Peijnenburg, W.; Quik, J.T.K.; et al. Considerations for Safe Innovation: The Case of Graphene. ACS Nano 2017, 11, 9574–9593. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Avitabile, E.; Bedognetti, D.; Ciofani, G.; Bianco, A.; Delogu, L.G. How can nanotechnology help the fight against breast cancer? Nanoscale 2018, 10, 11719–11731. [Google Scholar] [CrossRef] [Green Version]
- Viseu, T.; Lopes, C.M.; Fernandes, E.; Oliveira, M.; Lucio, M. A Systematic Review and Critical Analysis of the Role of Graphene-Based Nanomaterials in Cancer Theranostics. Pharmaceutics 2018, 10, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Feng, L.Z.; Liu, Z. Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy. Adv. Drug Deliv. Rev. 2016, 105, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.Z.; Liu, Z. Photothermally Enhanced Photodynamic Therapy Delivered by Nano-Graphene Oxide. ACS Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Muramatsu, H.; Hayashi, T.; Endo, M.; Terrones, M.; Dresselhaus, M.S. Fabrication of high-purity, double-walled carbon nanotube buckypaper. Chem. Vapor Depos. 2006, 12, 327–330. [Google Scholar] [CrossRef]
- Jiang, B.P.; Hu, L.F.; Shen, X.C.; Ji, S.C.; Shi, Z.J.; Liu, C.J.; Zhang, L.; Liang, H. One-Step Preparation of a Water-Soluble Carbon Nanohorn/Phthalocyanine Hybrid for Dual-Modality Photothermal and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2014, 6, 18008–18017. [Google Scholar] [CrossRef]
- Karahan, H.E.; Wiraja, C.; Xu, C.J.; Wei, J.; Wang, Y.L.; Wang, L.; Liu, F.; Chen, Y. Graphene Materials in Antimicrobial Nanomedicine: Current Status and Future Perspectives. Adv. Healthc. Mater. 2018, 7, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madannejad, R.; Shoaie, N.; Jahanpeyma, F.; Darvishi, M.H.; Azimzadeh, M.; Javadi, H. Toxicity of carbon-based nanomaterials: Reviewing recent reports in medical and biological systems. Chem. Biol. Interact. 2019, 307, 206–222. [Google Scholar] [CrossRef]
- Raja, I.S.; Song, S.J.; Kang, M.S.; Lee, Y.B.; Kim, B.; Hong, S.W.; Jeong, S.J.; Lee, J.C.; Han, D.W. Toxicity of Zero- and One-Dimensional Carbon Nanomaterials. Nanomaterials 2019, 9, 1214. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Zhang, X.X.; Sun, L.; Wei, Y.Q.; Wei, X.W. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 27. [Google Scholar] [CrossRef]
- Erol, O.; Uyan, I.; Hatip, M.; Yilmaz, C.; Tekinay, A.B.; Guler, M.O. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2433–2454. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Lin, J.; Wang, X.S.; Wang, Z.; Zhang, C.L.; He, M.; Wang, K.; Chen, F.; Li, Z.M.; Shen, G.X.; et al. Light-Triggered Theranostics Based on Photosensitizer-Conjugated Carbon Dots for Simultaneous Enhanced-Fluorescence Imaging and Photodynamic Therapy. Adv. Mater. 2012, 24, 5104–5110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beack, S.; Kong, W.H.; Jung, H.S.; Do, I.H.; Han, S.; Kim, H.; Kim, K.S.; Yun, S.H.; Hahn, S.K. Photodynamic therapy of melanoma skin cancer using carbon dot—Chlorin e6-hyaluronate conjugate. Acta Biomater. 2015, 26, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.W.; Wang, S.M.; Huang, Z.; Liu, J. The conjugates of carbon nanodots and chlorin e6 for enhancing cellular internalization and photodynamic therapy of cancers. Laser Phys. Lett. 2016, 13, 8. [Google Scholar] [CrossRef]
- Hu, T.Y.; He, J.; Zhang, S.M.; Mei, X.; Zhang, W.K.; Liang, R.Z.; Wei, M.; Evans, D.G.; Duan, X. An ultrathin photosensitizer for simultaneous fluorescence imaging and photodynamic therapy. Chem. Commun. 2018, 54, 5760–5763. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhi, X.; Hou, W.X.; Xia, F.F.; Zhang, J.P.; Li, L.X.; Hong, Y.P.; Yan, H.; Peng, C.; de la Fuentea, J.M.; et al. Gd3+-Ion-induced carbon-dots self-assembly aggregates loaded with a photosensitizer for enhanced fluorescence/MRI dual imaging and antitumor therapy. Nanoscale 2018, 10, 19052–19063. [Google Scholar] [CrossRef]
- Guan, M.R.; Ge, J.C.; Wu, J.Y.; Zhang, G.Q.; Chen, D.Q.; Zhang, W.; Zhang, Y.; Zou, T.J.; Zhen, M.M.; Wang, C.R.; et al. Fullerene/photosensitizer nanovesicles as highly efficient and clearable phototheranostics with enhanced tumor accumulation for cancer therapy. Biomaterials 2016, 103, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Rybkin, A.Y.; Belik, A.Y.; Goryachev, N.S.; Mikhaylov, P.A.; Kraevaya, O.A.; Filatova, N.V.; Parkhomenko, I.I.; Peregudov, A.S.; Terent’ev, A.A.; Larkina, E.A.; et al. Self-assembling nanostructures of water-soluble fullerene 60 -chlorin e6 dyads: Synthesis, photophysical properties, and photodynamic activity. Dyes Pigment. 2020, 180, 14. [Google Scholar] [CrossRef]
- Xiao, H.R.; Zhu, B.S.; Wang, D.L.; Pang, Y.; He, L.; Ma, X.F.; Wang, R.B.; Jin, C.Y.; Chen, Y.; Zhu, X.Y. Photodynamic effects of chlorin e6 attached to single wall carbon nanotubes through noncovalent interactions. Carbon 2012, 50, 1681–1689. [Google Scholar] [CrossRef]
- Marangon, I.; Menard-Moyon, C.; Silva, A.K.A.; Bianco, A.; Luciani, N.; Gazeau, F. Synergic mechanisms of photothermal and photodynamic therapies mediated by photosensitizer/carbon nanotube complexes. Carbon 2016, 97, 110–123. [Google Scholar] [CrossRef]
- Xie, L.S.; Wang, G.H.; Zhou, H.; Zhang, F.; Guo, Z.D.; Liu, C.; Zhang, X.Z.; Zhu, L. Functional long circulating single walled carbon nanotubes for fluorescent/photoacoustic imaging-guided enhanced phototherapy. Biomaterials 2016, 103, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.H.; Chen, D.P.; Zou, J.H.; Shao, J.J.; Tang, H.; Xu, H.; Si, W.L.; Dong, X.C. Tumor Microenvironment Responsive Oxygen-Self-Generating Nanoplatform for Dual-Imaging Guided Photodynamic and Photothermal Therapy. ChemistrySelect 2018, 3, 4366–4373. [Google Scholar] [CrossRef]
- Huang, P.; Xu, C.; Lin, J.; Wang, C.; Wang, X.S.; Zhang, C.L.; Zhou, X.J.; Guo, S.W.; Cui, D.X. Folic Acid-conjugated Graphene Oxide loaded with Photosensitizers for Targeting Photodynamic Therapy. Theranostics 2011, 1, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, S.J.; Wang, X.S.; Shen, G.X.; Lin, J.; Wang, Z.; Guo, S.W.; Cui, D.X.; Yang, M.; Chen, X.Y. Surface Functionalization of Chemically Reduced Graphene Oxide for Targeted Photodynamic Therapy. J. Biomed. Nanotechnol. 2015, 11, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Qin, H.M.; Amano, T.; Murakami, T.; Komatsu, N. Direct Fabrication of the Graphene-Based Composite for Cancer Phototherapy through Graphite Exfoliation with a Photosensitizer. ACS Appl. Mater. Interfaces 2015, 7, 23402–23406. [Google Scholar] [CrossRef]
- Zeng, Y.P.; Yang, Z.Y.; Luo, S.L.; Li, H.; Liu, C.; Hao, Y.H.; Liu, J.; Wang, W.D.; Li, R. Fast and facile preparation of PEGylated graphene from graphene oxide by lysosome targeting delivery of photosensitizer to efficiently enhance photodynamic therapy. RSC Adv. 2015, 5, 57725–57734. [Google Scholar] [CrossRef]
- Zeng, Y.P.; Luo, S.L.; Yang, Z.Y.; Huang, J.W.; Li, H.; Liu, C.; Wang, W.D.; Li, R. A folic acid conjugated polyethylenimine-modified PEGylated nanographene loaded photosensitizer: Photodynamic therapy and toxicity studies in vitro and in vivo. J. Mat. Chem. B 2016, 4, 2190–2198. [Google Scholar] [CrossRef]
- Cao, J.B.; An, H.Q.; Huang, X.L.; Fu, G.F.; Zhuang, R.Q.; Zhu, L.; Xie, J.; Zhang, F. Monitoring of the tumor response to nano-graphene oxide-mediated photothermal/photodynamic therapy by diffusion-weighted and BOLD MRI. Nanoscale 2016, 8, 10152–10159. [Google Scholar] [CrossRef]
- Shim, G.; Kim, M.G.; Jin, H.; Kim, J.; Oh, Y.K. Claudin 4-targeted nanographene phototherapy using a Clostridium perfringens enterotoxin peptide-photosensitizer conjugate. Acta Pharmacol. Sin. 2017, 38, 954–962. [Google Scholar] [CrossRef]
- Kim, D.J.; Kim, J.; Lee, H.L.; Lee, S.; Choi, J.S.; Kim, S.J.; Jeong, Y.I.; Kang, D.H. Redox-Responsive Nanocomposites Composed of Graphene Oxide and Chlorin e6 for Photodynamic Treatment of Cholangiocarcinoma. Bull. Korean Chem. Soc. 2018, 39, 1073–1082. [Google Scholar] [CrossRef]
- Kang, E.S.; Lee, T.H.; Liu, Y.; Han, K.H.; Lee, W.K.; Yoon, I. Graphene Oxide Nanoparticles Having Long Wavelength Absorbing Chlorins for Highly-Enhanced Photodynamic Therapy with Reduced Dark Toxicity. Int. J. Mol. Sci. 2019, 20, 4344. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |










| Entry | Carbon Nanomaterial | Properties | Advantages | Drawbacks |
|---|---|---|---|---|
| 1 | Carbon dot | D 1 = 0, strong optical absorption in the UV region (260–320 nm) | Low toxicity, excellent photoluminescence, good hydrophilicity, small size (below 10 nm), easy synthesis, good electrochemiluminescence, high stability in physiological media, good fluorescent property, biocompatible | Low solubility in physiological media, aggregation |
| 2 | Fullerene | D = 0, H 2 = mostly sp2, E.S.A. 3 = 80–90, T.C. 4 = 0.4, E.C. 5 = 10−10, T 6 = elastic, hardness = hard | Low toxicity, biocompatible | Low solubility in physiological media, aggregation |
| 3 | Carbon nanotube | D = 1, H = mostly sp2, E.S.A. = ~1300, T.C. = 3500, E.C. = structure-dependent, T = flexible, elastic, hardness = hard | Low toxicity, high conductivity, high chemical stability and sensitivity, high electron-transfer rate, biocompatible, strong NIR light absorption | Low solubility in physiological media, aggregation, low homogeneous size |
| 4 | Graphene | D = 2, H = sp2, E.S.A. = ~1500, T.C. = 4850–5300, E.C. = ~2000, T = flexible, elastic, hardness = uppermost | Low toxicity, high sensitivity, large surface area, inherent size- and shape-dependent optical properties, unique physicochemical behavior, biocompatible | Low solubility in physiological media, aggregation |
| Entry | Carbon Material | Concentration | Irradiation | Incubation Time | Cancer Cell Lines | Results | Ref |
|---|---|---|---|---|---|---|---|
| 1 | Chlorin e6–conjugated C-dots | 0–50 µM | 30 mW/cm2 (3 min) | 24 h | MGC803 cells | ~10% (cell viability) | [101] |
| 2 | Chlorin e6–carbon dot | 1 µM | 100 mW/cm2 (10 min) | 4 h | B16F10 cells | ~10% (cell viability) | [102] |
| 3 | Chlorin e6–polyethyleneimine-coated carbon nanodots | 2.6 μg/mL | 15.5 mW/cm2 (60 min) | 24 h | HeLa cancer cells | 20% (cell viability) | [103] |
| 4 | Layered double hydroxides–chlorin e6–carbon dots | 0–10 μg/mL | 27 J/cm2 | 24 h | HeLa cancer cells | 9.8% (cell viability) | [104] |
| 5 | Chlorin e6–carbon dot | 3.0 mg/kg body weight (b.w.) | 0.5 W/cm2 (10 min) | 24 h | BALB/c athymic nude mice (A549 cells) | Volume tumor was decreased (up to 80%) | [105] |
| Entry | Carbon Material | Concentration | Irradiation | Incubation Time | Cancer Cell Lines | Results | Ref |
|---|---|---|---|---|---|---|---|
| 1 | Fullerene (C70)–chlorin e6 | 0.05–0.2 mg/mL | 20 mW/cm2 at 660 nm (10 min) | 3 h | A549 cells | ~10% (cell viability) | [106] |
| 2 | Fullerene–chlorin e6 | 10 mM | 23 mW/cm2 at 630 nm (30 min) | 24 h | HeLa cancer cells | IC50 = 1.17 µM | [107] |
| Entry | Carbon Material | Concentration | Irradiation | Incubation Time | Cancer Cell Lines | Results | Ref |
|---|---|---|---|---|---|---|---|
| 1 | Single-wall carbon nanotubes–chlorin e6–chitosan | 5–100 µg/mL | 20 J/cm2 | 24 h | HeLa cells | ~10% cell viability at 30 µg/mL | [108] |
| 2 | Multi-walled carbon nanotubes–mTHPC | 8–20 µg/mL | 125 mW/cm2 at 650 nm (300 s) or 2.3W/cm2 at 808 nm (200 s) | 3 h | Human ovarian carcinoma SKOV-3 cells | ~10% (cell viability) | [109] |
| 3 | Chlorin e6 with albumin–single-walled carbon nanotube | 1–50 mg/mL | 0.15 W/cm2 at 630 nm (1 min) and 1 W/cm2 at 808 nm (2 min) | 12 h | Mouse squamous carcinoma cell line SCC-7 | 10% cell viability | [110] |
| 4 | MnO2-coated carbon nanotubes with chlorin e6 | 0.5–1 µg/mL | 1.0 W/cm2 at 660 nm (5 min) | 24 h | HeLa cells | IC50 of 0.58 mg/mL | [111] |
| Entry | Carbon Material | Concentration | Irradiation | Incubation Time | Cancer Cell Lines | Results | Ref |
|---|---|---|---|---|---|---|---|
| 1 | Graphene oxide–polyethylene glycol–chlorin e6 | 0.00138–0.011 mg/mL | 0.1 W/cm2 (10 min) | 24 h | Human nasopharyngeal epidermal carcinoma KB cell line | ~10% cell viability | [93] |
| 2 | Folic-acid-conjugated graphene oxide–chlorin e6 | 0–100 μM | ~30 mW/cm2 (10 min) | 48 h | MGC803 cells | ~10% cell viability | [112] |
| 3 | Graphene oxide– polyvinylpyrrolidone–chlorin e6 | 0–50 µM | 30 mW/cm2 (3 min) | 24 h | MGC803 cells | complete cell killing | [113] |
| 4 | Graphene–chlorin e6 | 0–0.20 µg/mL | 0.14 W/cm2 (2 min) | 24 h | HeLa cells | Up to 100% cell killing | [114] |
| 5 | Graphene oxide– polyethylene glycol–chlorin e6 | 0.25–2 µM | 0.2 W/cm2 (5 min) | 24 h | HeLa cells | 10% cell viability | [115] |
| 6 | Folic-acid-conjugated polyethylenimine–PEGylated graphene–chlorin e6 | 0.5–10 µg/mL | 200 W/cm2 (5 min) | 24 h | HeLa cells | ~15% cell viability | [116] |
| 7 | PEGylated nanographene–chlorin e6 | 0–2.0 µM | 0.1 W/cm2 (10 min) | 24 h | 4T1 cells | Complete cell killing | [117] |
| 8 | Graphene oxide– polyethylene glycol–chlorin e6 | 50 µmol/L | 1.5 W at 808 nm | 1 h | U87 cells | 10% cell viability | [118] |
| 9 | Chlorin e6–PEG-conjugated graphene oxide | 0–2.5 µg/mL | 2.0 J/cm2 | 24 h | CCA cells | 10% cell viability | [119] |
| 10 | Up-conversion nanoparticles–graphene oxide–chlorin e6 | 25–800 µg/mL | 0.72 W/cm2 (10 min) | 24 h | HeLa cells | 15% cell viability | [86] |
| 11 | Graphene oxide–chlorin e6 | 1.0 µM | 2 W/cm2 (15 min) | 3–24 h | A549 cells | IC50 = 0.69 at 3 h | [120] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, L.D.; Mfouo-Tynga, I.S. Learning from Nature: Bioinspired Chlorin-Based Photosensitizers Immobilized on Carbon Materials for Combined Photodynamic and Photothermal Therapy. Biomimetics 2020, 5, 53. https://0-doi-org.brum.beds.ac.uk/10.3390/biomimetics5040053
Dias LD, Mfouo-Tynga IS. Learning from Nature: Bioinspired Chlorin-Based Photosensitizers Immobilized on Carbon Materials for Combined Photodynamic and Photothermal Therapy. Biomimetics. 2020; 5(4):53. https://0-doi-org.brum.beds.ac.uk/10.3390/biomimetics5040053
Chicago/Turabian StyleDias, Lucas D., and Ivan S. Mfouo-Tynga. 2020. "Learning from Nature: Bioinspired Chlorin-Based Photosensitizers Immobilized on Carbon Materials for Combined Photodynamic and Photothermal Therapy" Biomimetics 5, no. 4: 53. https://0-doi-org.brum.beds.ac.uk/10.3390/biomimetics5040053