1. Introduction
Planar multi electrode arrays (pMEAs) based on thin film technology are widely used for the investigation of neuronal tissue [
1]. However, the established structuring methods are inappropriate for fulfilling the requirements driven by 3-dimensional cell culture technology since assembly in the third dimension is limited. This fact has initiated the development of 3-dimensional multi electrode arrays (3D MEAs) based on low temperature cofired ceramic (LTCC) technology [
2]. The approach allows the assembly of thick film electrodes encompassing a volume range of several hundred micrometers. The 3D MEA prototypes monitored primary rat hippocampal and cortical cell cultures over a period of 6 months [
2]. In previous works, two limiting usability aspects were unveiled: (i) the impedance of the used thick film gold electrodes (TFGe) increases strongly when the diameter decreases [
3] and (ii) optical observation is not possible at perpendicular electrodes.
The first mentioned aspect must be solved because small electrode diameters are needed to ensure adequate spatial resolution of recordings. Functionalization can improve the impedance characteristic [
4].
The second aspect causes problems in cell culture monitoring. Neuronal cells require several days until they start bursting [
5] and they can remain inactive under disadvantageous conditions. The reason for absent spikes can lie in inactivity or in poor contact of cells with the electrode. Since optical control of cell adhesion at the electrode is impossible, alternative methods are necessary to assess a good electrical contact and improve usability of the system.
A smart solution would be the use of the recording electrodes itself for the detection of cell attachment on their surface. In principle, impedance spectroscopy can tackle this issue. Biomass attached to an electrode entails a change of the local ionic environment and results in a parameter change of the constant phase element, which changes the impedance characteristic of electrodes [
6,
7,
8,
9]. The comparison of pristine gold electrodes with poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) coated ones revealed an impedance decrease, which enabled reliable cell growth detection [
10]. However, the cited works have used mesoscale electrodes or interdigital structures with huge area requirement that are not suitable for spatially resolved measurements. The present work aims to investigate the impedance change on thick film microelectrodes with diameters below 250 µm due to cell adhesion in order to assess their miniaturization capability. Since the impedance of a cell layer is in the range of some kΩ [
7], a first step towards the application would be a reduction of the initial electrode impedance. Electrodeposition of poly(3,4-ethylenedioxythiophene) (PEDOT) should decrease the impedance of TFGe [
11]. The suitability of pristine TFGe and such coated with PEDOT (PEDOTe) for adhesion detection were compared and the electrode size was varied in order to explore the miniaturization limits.
With the purpose of modelling different adhesion behavior independent from cell number, two human cell lines were selected. Hepatocellular carcinoma cell line (Hep-G2) is known to easily form a dense lawn on different surfaces. In contrast, the adhesion behavior of neuroblastoma glioma hybrid cell line (NG108-15) correlates with acidification of the medium during growth and surface properties [
12]. Culture conditions that lead to worse adhesion can thus be realized, for example, when the usual laminin coating is omitted [
13,
14]. In this case, the cells attach by forming globular cell clusters.
Since planar design facilitates the inspection of the electrodes, pMEAs containing either TFGe or PEDOTe were used. The used measurement system foreseen for neuronal recordings allows impedance measurements. The impedance of the microelectrodes with different diameter was captured over the culture time and possible electric parameters for adhesion detection were identified. The spatially assigned impedance increase was evaluated and compared with cell adhesion observed with a scanning electron microscope (SEM).
3. Results
3.1. Influence of Electrode Material and Conditioning on Impedance
Impedance characteristics of electrodes depend on the interface material. Gold is the most used electrode material in BioMEMS and has a Faradaic character, while the conducting polymer PEDOT is characterized by electronic and ionic conducting mechanisms [
15]. Impedance measurements represent the resulting variation of the charge transfer through the electrolyte-solid interface. Values measured at 1 kHz in PBS after 4 h storage (
Section 2.3.2) were classified according to electrode size. The resulting box plots on the base of all measured electrodes in
Figure 4 show that PEDOT coating decreases the electrode impedance by two orders of magnitude and weakens scaling effects significantly. The resulting noise reduction is a strong argument for the use of PEDOT functionalization as an interface material in biologic systems. Electropolymerization with PEDOT increases the miniaturization capability of thick film microelectrodes.
Electrode conditioning allows the electrochemical processes at the electrode surface to reach the equilibrium state. While the impedance characteristic of gold electrodes is dominated by the double layer capacitance [
16], ion diffusion is one significant influence on the impedance change of PEDOT coatings [
17]. The evaluation of conditioning influences on the electrode impedance provides crucial data for the assessment of the measurement accuracy.
Figure 5 depicts impedance data measured at 1 kHz before and after exposure to cell culture media. The values of all measured electrodes in the respective category are compared. Treatment cycles are described in
Section 2.3.2. The impedance ratio Z
dx/Z
0 is standardized to the value measured in cycle 1 (PBS after 4 h storage).
The results reveal that TFGe are subject to strong variations after medium exchange and during storage in cell culture medium for the first few days. The impedance change during conditioning can reach a ratio of 1.8 in comparison with the initial measurement in PBS. PEDOTe are more robust against such influences: the maximum ratio is below 1.4. The stronger sensitivity of TFGe to medium exposure over time can be attributed to pH changes or degradation of the cell culture medium during incubation. The highly polarizable double layer is prone to such changes. A further influence is the formation of monolayers on the gold surface [
16].
3.2. Influence of Cultivation Time on Impedance Characteristics
The impedance characteristic was studied over the cultivation time on the base of the daily measured data (
Section 2.3.4). The median values
of the impedance magnitude and
of the phase for data sets measured on TFGe and PEDOTe are presented as bode plots in
Figure 6. Data bases are the values of electrodes with 129 µm diameter.
A correlation between the impedance magnitude characteristic of TFGe and cultivation time is not evident. The phase course changes between day 1 and day 5.
On the contrary, the characteristic of PEDOTe reveals a clear tendency: the characteristic lines move evidently towards a higher impedance magnitude as cultivation time passes. An obvious trend occurs at frequencies above 100 Hz for both cell cultures. The phase course is not significantly affected. Since a closer examination of the results should give clearer insight, the ratio between initial impedance directly after cell seeding on the MEAs and the value after a defined cultivation time was studied.
3.3. Impedance Increase Dependent on Culture Time
Relating the measured impedance increase to the values captured on the day of cell seeding (day 3) eliminates conditioning influences. The median values
of the impedance magnitude measured at 1 kHz as a function on the cultivation time (
Section 2.3.4.) are the bases of the diagrams in
Figure 7. The measurement values of all electrode sizes are considered. The standardized relation
Zd/
Z3 uses the respective median
(d=x) with
x = day 3–7 and the reference value
3 measured at day 3 directly before cell seeding. Equation (1) gives the relation:
Referring to the impedance value
3 directly before cell seeding at day 3 compensates for conditioning effects.
Figure 7a compares the ratio obtained from impedance experiments with NG108-15 cells and
Figure 7b those with HepG2 cells.
The impedance ratio measured on gold does not change over the whole cultivation time in both experiments. This confirms the previous made statement based on the bode plots in
Figure 6a, that the impedance change of TFGe with small dimensions is not suitable for in situ monitoring of cell attachment.
PEDOT coating makes the increase in impedance due to cell attachment measurable. A large effect is evident. The less adherent cell culture NG108-15 shows a moderate impedance rise. On day 7, the ratio increases by a factor of 1.6. In comparison, the impedance of HepG2 cells increases strongly, in particular after day 6. The maximum ratio of 2.4 emerges on day 7.
At the end of the experiment, both cell lines still showed good viability on both MEA types. There was no significant influence of the electrode material on viability observed. The values varied between 76% and 82% after 4 days of cultivation. The total cell number of NG 108-15 cells was 305,350 ± 25% (TFG-MEAs) and 263,400 ± 2% (PEDOT-MEAs). HepG2 cells total number was 148,200 ± 3% (TFG-MEAs) and 151,300 ± 3% (PEDOT-MEAs). It must be noted here that the cell number does not correlate with the impedance increase. Although the number of NG108-15 cells on day 7 is evidently higher than that of HepG2, the impedance increase is significantly lower. This observation will be explained in the conclusions.
3.4. Impedance Increase on Individual Electrodes
The evident dependence of the impedance magnitude on culture time in a comfortable frequency range could be a valuable instrument for in situ monitoring of cell attachment on electrodes. Since TFGe are not sensitive enough, only data captured on PEDOTe were evaluated concerning their individual characteristics. The impedance data of two PEDOT-MEAs are evaluated for each cell culture. The individual electrode impedance on day 7 (Z7) is set in relation to that of day 3 (Z
3), similar to Equation (1).
Figure 8 depicts bar charts of this local impedance ratio Z7/Z3 dependent on the position of the single electrodes. Since the characteristics do not differ significantly, one example is presented for each cell culture in
Figure 8. The columns in the graph are assigned to electrodes with equal diameter, rows include electrodes with different diameter (compare
Figure 1d). The impedance ratio observed on individual electrodes in cell culture NG108-15 is lower than 2.75. That of cell culture HepG2 is evidently higher and can reach values above 3.5.
Overview SEM images are provided in
Figure S1 (NG108-15) and Figure S2 (HepG2) as Supplementary Material. The observed cell spreading matches well with the impedance maps in
Figure 8.
Figure 9 shows selected SEM micrographs.
Figure 9a depicts an example of an electrode with agglomerates of NG108-15 cells. The image is representative for a good electrode coverage in this study. In general, cells adhesion on the surface is poor and only a few electrodes are covered with more than single cell clusters. The low to moderate local impedance increase correlates well with the impressions gained from SEM micrographs. The maximum value of the impedance ration on single electrodes amounts to 2.65 and was observed on a 129-µm-electrode.
The cell lawn of the HepG2 culture spread well over the surface; only a few electrodes were left uncovered. Some electrodes were partially overgrown as demonstrated in the example in
Figure 9b and some are fully covered with a dense cell lawn as shown in the example in
Figure 9c. The observed overgrowth correlates with the local impedance ratio. The maximum value of the impedance ratio was 6.8 and occurred on the smallest electrodes with a diameter of 86 µm.
4. Discussion and Conclusions
The impedance characteristics of TFGe and PEDOTe are compared in the first part of this work. The results in
Figure 4 show that PEDOT coating entails a significant reduction of the impedance magnitude. The impedance magnitude reached values in the range of some kΩ. In addition to the conducting mechanism, the rough surface structure (see
Figure 2) is assumed to increase the effective electrode area and contributes to this decrease. Measurements after storage in cell culture media revealed that PEDOTe were less prone to conditioning influences than TFGe (
Figure 5).
Since the impedance change caused by cell overgrowth was in the kΩ-range [
7], it is plausible that cell tissue related impedance changes on TFG-MEAs in
Figure 6a,b were not detectable because of the high initial impedance of TFGe, which was around 20–70 kΩ at 1 kHz, depending on size (see
Figure 4). The impedances of PEDOTe amounted to 2–5 kΩ and were in the order of magnitude of the cell related changes. Curves measured with PEDOT-MEAs (
Figure 6c,d) reveal a clear tendency. Therefore, only the results based on PEDOT-MEAs were considered in
Section 3.4.
In accordance with the literature [
10], the ratio Zd/Z3 at 1 kHZ was a measure for the decreasing portion of the electrode area in direct contact with the electrolyte when cells cover the surface.
Figure 7 reveals different characteristics dependent on cell type. From the electrical point of view, only cells with tight contact to the electrode contribute to an impedance increase.
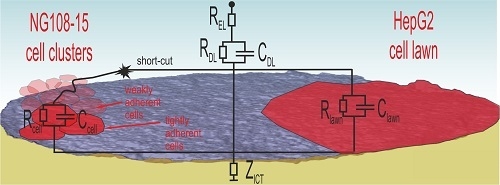
Figure 10 illustrates the situation for both cell types.
NG108-15 cells form globular cell clusters. Some clusters adhere tightly at the electrode while others attach weakly on top. These cells contribute to the cell number, but the weak contact enables short-cuts through the surrounding electrolyte. Consequently, the cell number does not correlate with the impedance measurement. The SEM investigation confirms the impedance measurement because the PBS washing steps during preparation removes weakly adherent cells and only the tightly adherent ones, which contribute to impedance changes, remain at the surface.
In the case of the well-spreading HepG2 culture, the cell lawn covers wide areas and electrodes, many of them completely. The dense lawn adheres strongly and is not washed away during SEM preparation. The observed impedance increase in
Figure 8b is related to the covered electrode area.
Since the cell number in this experiment is lower than that made with NG108-15, we attribute the impedance increase to the cell adhesion at the electrode. The impression based on SEM pictures (
Figures S1 and S2) matches well with the spatial distribution of the impedance ratio in
Figure 8.
These promising results must be underpinned by pursuing investigations. Aspects of long-term stability must be tested. Functionality improvement, e.g., by the use of composites of carbon nanotubes and PEDOT (PEDOT-CNT) is a further task. Electropolymerized PEDOT, here tested on pMEAS, can be applied on 3D MEAs in future designs.
The current test shows that the impedance increase caused by cell adhesion is large enough to be detected by PEDOTe, which can be used for signal recordings at the same time. Since the impedance of the thick film microelectrodes with a diameter of 86 µm still did not reach the operation limits of the electronic measurement system, their dimensions can be further decreased. The measurement equipment used for recordings is able to detect the changes, which reached a factor of 6.8 in this experiment. The crucial usability benefit consists in the possibility of testing the tight cell contact with the electronic system prior to the expected start of bursting, saving experimental time and money in the case of failure.