Blood Serum Cytokines in Patients with Subacute Spinal Cord Injury: A Pilot Study to Search for Biomarkers of Injury Severity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants Enrollment
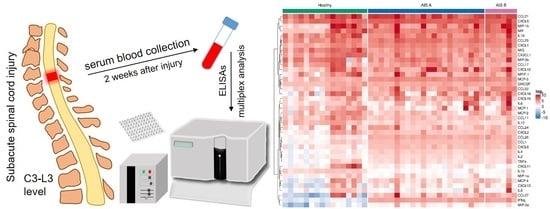
2.2. Sample Acquisition
2.3. Biochemical Analysis
2.4. Statistical Analysis
3. Results
3.1. Subject Demographics
3.2. Multiplex Analysis of Blood Serum Cytokines
3.3. Determination of the Effect of the Spinal Cord Injury Region on Blood Serum Cytokines
3.4. Classifying Injury Severity with Blood Serum Cytokines
3.5. NSE and VEGF Blood Serum Concentration
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [Green Version]
- Albayar, A.A.; Roche, A.; Swiatkowski, P.; Antar, S.; Ouda, N.; Emara, E.; Smith, D.H.; Ozturk, A.K.; Awad, B.I. Biomarkers in Spinal Cord Injury: Prognostic Insights and Future Potentials. Front. Neurol. 2019, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Kwon, B.K.; Bloom, O.; Wanner, I.-B.; Curt, A.; Schwab, J.M.; Fawcett, J.; Wang, K.K. Neurochemical biomarkers in spinal cord injury. Spinal Cord 2019, 57, 819–831. [Google Scholar] [CrossRef]
- Okada, S. The pathophysiological role of acute inflammation after spinal cord injury. Inflamm. Regen. 2016, 36, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figley, S.A.; Khosravi, R.; Legasto, J.M.; Tseng, Y.-F.; Fehlings, M.G. Characterization of Vascular Disruption and Blood–Spinal Cord Barrier Permeability following Traumatic Spinal Cord Injury. J. Neurotrauma 2014, 31, 541–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.L.; Agarwal, N.; Barrese, J.C.; Heary, R.F. Current therapeutic strategies for inflammation following traumatic spinal cord injury. Neural Regen. Res. 2012, 7, 1812–1821. [Google Scholar] [PubMed]
- Anwar, M.A.; Al Shehabi, T.S.; Eid, A.H. Inflammogenesis of Secondary Spinal Cord Injury. Front. Cell. Neurosci. 2016, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Rust, R.; Kaiser, J. Insights into the Dual Role of Inflammation after Spinal Cord Injury. J. Neurosci. 2017, 37, 4658–4660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Y.; Shultz, R.B. Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury. Neural Regen. Res. 2017, 12, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Tator, C.H. Review of treatment trials in humanspinal cord injury. Neurosurgery 2006, 59, 957–987. [Google Scholar] [CrossRef]
- Kwon, B.K.; Elizei, S.S. The translational importance of establishing biomarkers of human spinal cord injury. Neural Regen. Res. 2017, 12, 385–388. [Google Scholar] [CrossRef]
- Kwon, B.K.; Stammers, A.M.; Belanger, L.M.; Bernardo, A.; Chan, D.; Bishop, C.M.; Slobogean, G.P.; Zhang, H.; Umedaly, H.; Giffin, M.; et al. Cerebrospinal Fluid Inflammatory Cytokines and Biomarkers of Injury Severity in Acute Human Spinal Cord Injury. J. Neurotrauma 2010, 27, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedshina, Y.O.; Akhmetzyanova, E.R.; Martynova, E.V.; Khaiboullina, S.F.; Galieva, L.R.; Rizvanov, A.A. Systemic and Local Cytokine Profile following Spinal Cord Injury in Rats: A Multiplex Analysis. Front. Neurol. 2017, 8, 581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, E.; Aguilar-Cevallos, J.; Silva-Garcia, R.; Ibarra, A. Cytokine and Growth Factor Activation In Vivo and In Vitro after Spinal Cord Injury. Mediat. Inflamm. 2016, 2016, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalkilic, T.; Fallah, N.; Noonan, V.K.; Elizei, S.S.; Dong, K.; Belanger, L.; Ritchie, L.; Tsang, A.; Bourassa-Moreau, E.; Heran, M.K.; et al. Predicting Injury Severity and Neurological Recovery after Acute Cervical Spinal Cord Injury: A Comparison of Cerebrospinal Fluid and Magnetic Resonance Imaging Biomarkers. J. Neurotrauma 2018, 35, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Yokobori, S.; Zhang, Z.; Moghieb, A.; Mondello, S.; Gajavelli, S.; Dietrich, W.D.; Bramlett, H.; Hayes, R.L.; Wang, M.; Wang, K.K.; et al. Acute Diagnostic Biomarkers for Spinal Cord Injury: Review of the Literature and Preliminary Research Report. World Neurosurg. 2015, 83, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Prakash, J.; Singh, R.; Verma, A.; Bansal, H. Nitric Oxide Metabolite Concentration in Cerebrospinal Fluid: Useful as a Prognostic Marker? Asian Spine J. 2016, 10, 828–833. [Google Scholar] [CrossRef] [Green Version]
- Moghieb, A.; Bramlett, H.M.; Das, J.H.; Yang, Z.; Selig, T.; Yost, R.A.; Wang, M.S.; Dietrich, W.D.; Wang, K.K.W. Differential Neuroproteomic and Systems Biology Analysis of Spinal Cord Injury. Mol. Cell. Proteom. 2016, 15, 2379–2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streijger, F.; Skinnider, M.A.; Rogalski, J.C.; Balshaw, R.; Shannon, C.P.; Prudova, A.; Belanger, L.; Ritchie, L.; Tsang, A.; Christie, S.; et al. A Targeted Proteomics Analysis of Cerebrospinal Fluid after Acute Human Spinal Cord Injury. J. Neurotrauma 2017, 34, 2054–2068. [Google Scholar] [CrossRef]
- Zha, Z.; Bucher, F.; Nejatfard, A.; Zheng, T.; Zhang, H.; Yea, K.; Lerner, R.A. Interferon-γ is a master checkpoint regulator of cytokine-induced differentiation. Proc. Natl. Acad. Sci. USA 2017, 114, E6867–E6874. [Google Scholar] [CrossRef] [Green Version]
- Stubbs, V.E.L.; Power, C.; Patel, K.D. Regulation of eotaxin-3/CCL26 expression in human monocytic cells. Immunology 2010, 130, 74–82. [Google Scholar] [CrossRef]
- Khaiboullina, S.F.; Gumerova, A.R.; Khafizova, I.F.; Martynova, E.V.; Lombardi, V.C.; Bellusci, S.; Rizvanov, A.A. CCL27: Novel Cytokine with Potential Role in Pathogenesis of Multiple Sclerosis. BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kil, K.; Zang, Y.C.; Yang, D.; Markowski, J.; Fuoco, G.S.; Vendetti, G.C.; Rivera, V.M.; Zhang, J.Z. T cell responses to myelin basic protein in patients with spinal cord injury and multiple sclerosis. J. Neuroimmunol. 1999, 98, 201–207. [Google Scholar] [CrossRef]
- Ankeny, D.P.; Popovich, P.G. Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury. Neuroscience 2009, 158, 1112–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biglari, B.; Swing, T.; Child, C.; Büchler, A.; Westhauser, F.; Bruckner, T.; Ferbert, T.; Gerner, H.J.; Moghaddam, A. A pilot study on temporal changes in IL-1β and TNF-α serum levels after spinal cord injury: The serum level of TNF-α in acute SCI patients as a possible marker for neurological remission. Spinal Cord 2015, 53, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.L.; Hayes, K.C.; Dekaban, G.A. Clinical Correlates of Elevated Serum Concentrations of Cytokines and Autoantibodies in Patients with Spinal Cord Injury. Arch. Phys. Med. Rehabilit. 2007, 88, 1384–1393. [Google Scholar] [CrossRef]
- Stein, A.; Panjwani, A.; Sison, C.; Rosen, L.; Chugh, R.; Metz, C.; Bank, M.; Bloom, O. Pilot Study: Elevated Circulating Levels of the Proinflammatory Cytokine Macrophage Migration Inhibitory Factor in Patients with Chronic Spinal Cord Injury. Arch. Phys. Med. Rehabilit. 2013, 94, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.R.S.; Foster, G.R.; Leung, S.; Leaman, D.; Stark, G.R.; Ransohoff, R.M. Characterization of β-R1, a Gene That Is Selectively Induced by Interferon β (IFN-β) Compared with IFN-α. J. Biol. Chem. 1996, 271, 22878–22884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassanshahi, G.; Jafarzadeh, A.; Esmaeilzadeh, B.; Arababadi, M.K.; Yousefi, H.; Dickson, A.J. Assessment of NK Cells Response to Hepatocyte Derived Chemotactic Agents. Pak. J. Biol. Sci. 2008, 11, 1120–1125. [Google Scholar] [CrossRef] [Green Version]
- Franciotta, D.; Martino, G.; Zardini, E.; Furlan, R.; Bergamaschi, R.; Andreoni, L.; Cosi, V. Serum and CSF levels of MCP-1 and IP-10 in multiple sclerosis patients with acute and stable disease and undergoing immunomodulatory therapies. J. Neuroimmunol. 2001, 115, 192–198. [Google Scholar] [CrossRef]
- Szczuciński, A.; Kalinowska, A.; Losy, J. CXCL11 (Interferon-Inducible T-Cell Alpha Chemoattractant) and Interleukin-18 in Relapsing-Remitting Multiple Sclerosis Patients Treated with Methylprednisolone. Eur. Neurol. 2007, 58, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-J.; Cao, D.-L.; Zhang, X.; Ji, R.-R.; Gao, Y.-J. Chemokine contribution to neuropathic pain: Respective induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons. Pain 2013, 154, 2185–2197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, H.; Fu, X.; Ma, L.; Sun, H.; Chen, G.; Song, M.; Chen, W.; Chen, Y.; Tan, M.; Guo, Y.; et al. The chemokine CXCL1 and its receptor CXCR2 contribute to chronic stress-induced depression in mice. FASEB J. 2019, 33, 8853–8864. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.G.; Diamond, M.L.; Boles, J.A.; Berger, R.P.; Tisherman, S.A.; Kochanek, P.M.; Wagner, A.K. Acute CSF interleukin-6 trajectories after TBI: Associations with neuroinflamma-tion, polytrauma, and outcome. Brain Behav. Immun. 2015, 45, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Ahadi, R.; Khodagholi, F.; Daneshi, A.; Vafaei, A.; Mafi, A.A.; Jorjani, M. Diagnostic Value of Serum Levels of GFAP, pNF-H, and NSE Compared with Clinical Findings in Severity Assessment of Human Traumatic Spinal Cord Injury. Spine 2015, 40, E823–E830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieder, M.D.M.; Oses, J.P.; Kutchak, F.M.; Sartor, M.; Cecchini, A.; Rodolphi, M.S.; Wiener, C.D.; Kopczynski, A.; Muller, A.P.; Strogulski, N.R.; et al. Serum Biomarkers and Clinical Outcomes in Traumatic Spinal Cord Injury: Prospective Cohort Study. World Neurosurg. 2019, 122, e1028–e1036. [Google Scholar] [CrossRef]
- Schwartz, K.; Henzel, M.K.; Richmond, M.A.; Zindle, J.K.; Seton, J.M.; Lemmer, D.P.; Alvarado, N.; Bogie, K.M. Biomarkers for recurrent pressure injury risk in persons with spinal cord injury. J. Spinal Cord Med. 2020, 43, 696–703. [Google Scholar] [CrossRef]



| Characteristics | AIS Grade A | AIS Grade B |
|---|---|---|
| No. of subjects | 22 | 6 |
| Gender (Male/Female) | 16/6 | 3/3 |
| Age | 40.2 ± 13.9 | 32.5 ± 6.3 |
| Etiology | ||
| traffic accident | 10 | 1 |
| fall | 10 | 3 |
| other | 2 | 2 |
| Regions of lesion | ||
| cervical | 13 | 0 |
| thoracic | 9 | 3 |
| lumbar | 0 | 3 |
| Comorbid and Proinflammatory Conditions Numbers | Concurrent Medications Numbers | ||
|---|---|---|---|
| Urinary tract infections Interstitial cystitis | 0 1 (AIS A) | Intensive therapy * anti-inflammatory medications Dexamethasone 4.0 i/m № 7 (3 days) | 28 (22 AIS A, 6 AIS B) |
| Community-acquired pneumonia | 1 (AIS A) | spasmolytic drugs Spasmalin 5.0 i/m once a day (5 days) Tizanidine 12 mg once a day (10 days) | 5 (AIS A) 1 (AIS A) |
| Post-traumatic pneumonia | 2 (AIS A) | analgesics Ketorol 1.0 i/m once a day (5 days) Tramadol 50 i/m once a day (2 days) | 23 (17 AIS A, 6 AIS B) 5 (AIS A) |
| Spondyloarthrosis | 1 (AIS A) | Supportive therapy ** Anticoagulants Enixum 0.4 once a day | 24 (20 AIS A, 4 AIS B) |
| Smoker | 5 (4 AIS A, 1 AIS B) | Antibiotics Levofloxacin 500 mg once a day Ceftriaxone 2.0 once a day | 1 (AIS A) 2 (AIS A) |
| Hypertensive heart disease | 1 (AIS B) | Hypertension Amlodipine 5 mg once a day | 1 (AIS B) |
| Markers | Uninjured Control | C | Th | L |
|---|---|---|---|---|
| CXCL1 | 26.80 (20.21) | 47.96 (18.06) | 39.12 (13.17) | 31.54 (3.55) |
| 18.16 (13.23‒40.48) | 46.08 (34.55‒59.12) * | 34.23 (29.90‒45.37) | 31.54 (30.29‒32.80) | |
| CXCL10 | 10.76 (17.69) | 26.58 (31.55) | 15.16 (14.05) | 5.10 (0.13) |
| 4.26 (1.79‒9.72) | 15.92 (6.98‒26.43) * | 9.36 (5.93‒19.52) | 5.10 (5.05‒5.14) | |
| CXCL11 | 72.70 (175.50) | 2.01 (1.62) | 2.39 (1.08) | 1.00 (0.02) |
| 2.02 (1.21‒36.61) | 1.52 (1.11‒1.97) * | 2.12 (1.80‒2.71) | 1.00 (1.00‒1.01) | |
| MIG | 114.44 (154.44) | 31.74 (19.65) | 27.13 (11.64) | 15.27 (7.63) |
| 70.53 (40.30‒136.70) | 27.13 (16.55‒44.95) * | 26.45 (18.75‒36.73) * | 15.27 (12.57‒17.96) | |
| CCL22 | 10.41 (9.80) | 59.53 (149.58) | 24.67 (17.01) | 47.52 (2.55) |
| 7.10 (3.43‒14.98) | 15.77 (7.56‒20.01) | 16.87 (10.59‒37.67) * | 47.52 (46.62‒48.42) * | |
| CCL26 | 2.00 (1.46) | 15.30 (6.07) | 14.54 (7.50) | 8.29 (1.51) |
| 1.38 (0.92‒2.46) | 13.19 (11.11‒16.84) # | 12.02 (10.81‒14.72) # | 8.29 (7.75‒8.82) ** | |
| CXCL6 | 2.61 (2.25) | 8.88 (3.19) | 8.01 (2.42) | 6.60 (0.54) |
| 2.07 (0.80‒3.11) | 7.58 (7.47‒10.11) # | 7.22 (6.98‒8.62) # | 6.60 (6.40‒6.79) * | |
| CCL1 | 3.25 (2.62) | 9.63 (3.47) | 7.88 (0.87) | 7.30 (0.40) |
| 1.90 (1.40‒4.96) | 8.55 (7.78‒9.53) # | 7.92 (7.58‒8.36) # | 7.30 (7.16‒7.44) * | |
| IFNγ | 0.42 (0.20) | 20.44 (6.00) | 20.13 (6.46) | 13.86 (3.42) |
| 0.39 (0.24‒0.60) | 18.98 (17.10‒21.03) # | 17.38 (16.27‒23.45) # | 13.86 (12.65‒15.06) # | |
| IL10 | 12.40 (7.81) | 6.41 (5.85) | 3.77 (1.43) | 2.71 (0.13) |
| 12.13 (6.47‒15.23) | 4.97 (3.79‒6.02) * | 3.27 (2.74‒4.99) ** | 2.71 (2.67‒2.76) * | |
| IL1b | 5.08 (1.67) | 1.69 (1.37) | 1.09 (0.46) | 0.77 (0.08) |
| 5.32 (3.77‒6.51) | 1.16 (1.00‒1.68) # | 0.93 (0.87‒1.02) # | 0.77 (0.74‒0.80) # | |
| IL4 | 1.58 (1.21) | 5.87 (2.12) | 4.66 (1.18) | 3.93 (0.61) |
| 0.99 (0.82‒2.25) | 4.87 (4.55‒6.60) # | 4.75 (3.81‒5.25) # | 3.93 (3.71‒4.15) ** | |
| MCP-3 | 10.28 (5.16) | 42.35 (14.34) | 37.33 (9.59) | 27.09 (7.38) |
| 12.02 (6.15‒13.56) | 35.93 (34.13‒50.99) # | 37.09 (30.44‒44.48) # | 27.09 (24.48‒29.70) * |
| Markers | Uninjured Control | SCI | AIS A | AIS B |
|---|---|---|---|---|
| NSE | 1.54 (0.80) | 3.31 (3.15) | 2.85 (2.51) | 4.74 (4.61) |
| 1.40 (1.10‒1.62) | 2.10 (1.50‒3.70) * | 2.05 (1.52‒3.38) | 3.00 (1.50‒6.20) | |
| VEGF | 233.10 (220.59) | 373.03 (302.61) | 368.27 (282.24) | 368.27 (282.24) |
| 179.54 (75.84‒356.80) | 324.08 (107.85‒592.85) | 344.73 (111.05‒587.15) | 344.73 (111.05‒587.15) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogurcov, S.; Shulman, I.; Garanina, E.; Sabirov, D.; Baichurina, I.; Kuznetcov, M.; Masgutova, G.; Kostennikov, A.; Rizvanov, A.; James, V.; et al. Blood Serum Cytokines in Patients with Subacute Spinal Cord Injury: A Pilot Study to Search for Biomarkers of Injury Severity. Brain Sci. 2021, 11, 322. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci11030322
Ogurcov S, Shulman I, Garanina E, Sabirov D, Baichurina I, Kuznetcov M, Masgutova G, Kostennikov A, Rizvanov A, James V, et al. Blood Serum Cytokines in Patients with Subacute Spinal Cord Injury: A Pilot Study to Search for Biomarkers of Injury Severity. Brain Sciences. 2021; 11(3):322. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci11030322
Chicago/Turabian StyleOgurcov, Sergei, Iliya Shulman, Ekaterina Garanina, Davran Sabirov, Irina Baichurina, Maxim Kuznetcov, Galina Masgutova, Alexander Kostennikov, Albert Rizvanov, Victoria James, and et al. 2021. "Blood Serum Cytokines in Patients with Subacute Spinal Cord Injury: A Pilot Study to Search for Biomarkers of Injury Severity" Brain Sciences 11, no. 3: 322. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci11030322