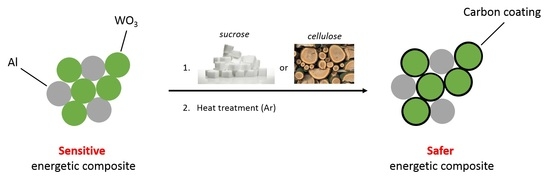
Mechanical Desensitization of an Al/WO3 Nanothermite by Means of Carbonaceous Coatings Derived from Carbohydrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tungsten (VI) Oxide/Carbon Composites Synthesis
2.2. Nanothermite Preparation
2.3. Characterization Techniques
3. Results and Discussion
3.1. Characterization of the Saccharide-Derived Carbons and the WO3/C Composites
3.2. Sensitivities of the Al/WO3/C Nanothermites
3.3. Combustion of the Al/WO3/C Nanothermites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fischer, S.H.; Grubelich, M.C. Theoretical energy release of thermites, intermetallics, and combustible metals. In Proceedings of the 24th International Pyrotechnics Seminar, Monterey, CA, USA, 27–31 July 1998; pp. 231–286. [Google Scholar]
- Pantoya, M.L.; Granier, J.J. Combustion behavior of highly energetic thermites: Nano versus micron composites. Propellants Explos. Pyrotech. 2005, 30, 53–62. [Google Scholar] [CrossRef]
- Valliapan, S.; Swiatkiewicz, J.; Puszynski, J.A. Reactivity of aluminum nanopowders with metal oxides. Powder Technol. 2005, 156, 164–169. [Google Scholar] [CrossRef]
- Sanders, V.E.; Asay, B.W.; Foley, T.J.; Tappan, B.C.; Pacheco, A.N.; Son, S.F. Reaction propagation of four nanoscale energetic composites (Al/MoO3, Al/WO3, Al/CuO and Bi2O3). J. Propul. Power 2007, 23, 707–714. [Google Scholar] [CrossRef]
- Puszynski, J.A. Processing and characterization of aluminum-based nanothermites. J. Therm. Anal. Calorim. 2009, 96, 677–685. [Google Scholar] [CrossRef]
- Sun, J.; Pantoya, M.L.; Simon, S.L. Dependence of size and size distribution on reactivity of aluminum nanoparticles in reactions with oxygen and MoO3. Thermochim. Acta 2006, 444, 117–127. [Google Scholar] [CrossRef]
- Dutro, G.M.; Yetter, R.A.; Risha, G.A.; Son, S.F. The effect of stoichiometry on the combustion behavior of a nanoscale Al/MoO3 thermite. Proc. Combust. Inst. 2009, 32, 1921–1928. [Google Scholar] [CrossRef]
- Pantoya, M.L.; Levitas, V.I.; Granier, J.J.; Henderson, J.B. Effect of bulk density on reaction propagation in nanothermites and micron thermites. J. Propul. Power 2009, 25, 465–470. [Google Scholar] [CrossRef]
- Comet, M.; Pichot, V.; Siegert, B.; Schnell, F.; Ciszek, F.; Spitzer, D. Phosphorous-based nanothermites: A new generation of energetic materials. J. Phys. Chem. Solids 2010, 71, 64–68. [Google Scholar] [CrossRef]
- Comet, M.; Schnell, F.; Pichot, V.; Mory, J.; Risse, B.; Spitzer, D. Boron as fuel for ceramic thermites. Energy Fuels 2014, 28, 4139–4148. [Google Scholar] [CrossRef]
- Dixon, G.P.; Martin, J.A.; Thompson, D. Lead-Free Percussion Primer Mixes Based on Metastable Interstitial Composite (MIC) Technology. U.S. Patent 5,717,159, 10 February 1998. [Google Scholar]
- Wang, L.; Luss, D.; Martirosyan, K.S. The behavior of nanothermite reaction based on Bi2O3/Al. J. Appl. Phys. 2011, 110, 074311. [Google Scholar] [CrossRef]
- Shende, R.; Subramanian, S.; Hasan, S.; Apperson, S.; Thiruvengadathan, R.; Gangopadyay, R.; Gangopadhyay, S. Nanoenergetic composites of CuO nanorods, nanowires, and Al-nanoparticles. Propellants Explos. Pyrotech. 2008, 33, 122–130. [Google Scholar] [CrossRef]
- Gibot, P.; Comet, M.; Vidal, L.; Moitrier, F.; Lacroix, F.; Suma, Y.; Schnell, F.; Spitzer, D. Synthesis of WO3 nanoparticles for superthermites by the template method from silica spheres. Solid State Sci. 2011, 13, 908–914. [Google Scholar] [CrossRef]
- United Nations. Recommendations on the Transport of Dangerous Goods: Manual of Tests and Criteria, 4th revised ed.; United Nations: New York, NY, USA; Geneva, Switzerland, 2007. [Google Scholar]
- Steelman, R.; Clark, B.; Pantoya, M.L.; Heaps, R.J. Desensitizing nanopowders to electrostatic discharge ignition. J. Electrost. 2015, 76, 102–107. [Google Scholar] [CrossRef]
- Poper, K.H.; Collins, E.S.; Pantoya, M.L.; Daniels, M.A. Controlling the electrostatic discharge ignition sensitivity of composite energetic materials using carbon nanotube additives. J. Electrost. 2014, 72, 428–432. [Google Scholar] [CrossRef]
- Foley, T.; Pacheco, A.; Malchi, J.; Yetter, R.; Higa, K. Development of nanothermite composites with variable electrostatic discharge ignition thresholds. Propellants Explos. Pyrotech. 2007, 32, 431–434. [Google Scholar] [CrossRef]
- Bach, A.; Gibot, P.; Vidal, L.; Gadiou, R.; Spitzer, D. Modulation of the Reactivity of a WO3/Al Energetic Material with Graphitized Carbon Black as Additive. J. Energet. Mater. 2015, 33, 260–276. [Google Scholar] [CrossRef]
- Gibot, P.; Bach, A.; Vidal, L.; Schnell, F.; Gadiou, R.; Spitzer, D. Safer and performing energetic materials based on polyaniline-doped nanocomposites. J. Energet. Mater. 2017, 35, 136–147. [Google Scholar] [CrossRef]
- Collins, E.S.; Gesner, J.P.; Pantoya, M.L.; Daniels, M.A. Synthesizing aluminium particles towards controlling electrostatic discharge ignition sensitivity. J. Electrost. 2014, 72, 28–32. [Google Scholar] [CrossRef]
- Weir, C.; Pantoya, M.L.; Daniels, M.A. The role of aluminum particle size in electrostatic ignition sensitivity of composite energetic materials. Combust. Flame 2013, 160, 2279–2281. [Google Scholar] [CrossRef]
- Kelly, D.G.; Beland, P.; Brousseau, P.; Petre, C.F. Formation of additive-containing nanothermites and modifications to their sensitivity. J. Energet. Mater. 2016, 35, 331–345. [Google Scholar] [CrossRef]
- Siegert, B.; Comet, M.; Muller, O.; Pourroy, G.; Spitzer, D. Reduced-sensitivity Nanothermites containing manganese oxide filled carbon nanofibers. J. Phys. Chem. C 2010, 114, 19562–19568. [Google Scholar] [CrossRef]
- Collins, E.S.; Skelton, B.R.; Pantoya, M.L.; Irin, F.; Green, M.J. Ignition sensitivity and electrical conductivity of an aluminum fluoropolymer reactive material with carbon nanofillers. Combust. Flame 2015, 162, 1417–1421. [Google Scholar] [CrossRef]
- Kappagantula, K.; Pantoya, M.L.; Hunt, E.M. Impact ignition of aluminum-teflon based energetic materials impregnated with nano-structured carbon additives. J. Appl. Phys. 2012, 112, 024902. [Google Scholar] [CrossRef]
- Kappagantula, K.; Pantoya, M.L. Experimentally measured thermal transport properties aluminum-polytetrafluoroethylene nanocomposites with graphene and carbon nanotube additives. Int. J. Heat Mass Transfer. 2012, 55, 817–824. [Google Scholar] [CrossRef]
- Madhav Reddy, K.; Rao, T.N.; Radha, K.; Joardar, J. Nanostructured tungsten carbides by thermochemical processing. J. Alloys Compd. 2010, 494, 404–409. [Google Scholar] [CrossRef]
- Inagaki, M.; Kang, F. Materials Science and Engineering of Carbon: Fundamentals, 2nd ed.; Tsinghua University Press Limited, Elsevier Inc.: Amsterdam, The Netherlands, 2014; Chapter 2. [Google Scholar]
- Tang, M.M.; Bacon, R. Carbonization of cellulose fibers–Low temperature pyrolysis. Carbon 1964, 2, 211–220. [Google Scholar] [CrossRef]
- NATO. STANAG 4489. Standardization Agreement (STANAG) on Explosives, Impact Sensitivity Tests; NATO Standardization Agency: Brussels, Belgium, 1999. [Google Scholar]
- NATO. STANAG 4487. Standardization Agreement (STANAG) on Explosives, Friction Sensitivity Tests; NATO Standardization Agency: Brussels, Belgium, 2002. [Google Scholar]
- NATO. STANAG 4490. Standardization Agreement (STANAG) on Explosives, Electrostatic Discharge Sensitivity Tests; NATO Standardization Agency: Brussels, Belgium, 2001. [Google Scholar]
- Wang, N.; Yao, B.D.; Chan, Y.F.; Zhang, X.Y. Enhanced photothermal effect in Si nanowires. Nano Lett. 2003, 3, 475–477. [Google Scholar] [CrossRef]
- Ohkura, Y.; Rao, P.M.; Zheng, X. Flash ignition of Al nanoparticles: Mechanism and applications. Combust. Flame 2011, 158, 2544–2548. [Google Scholar] [CrossRef]
- Piercy, D.G.; Klapotke, T.M. Nanoscale aluminum–metal oxide (thermite) reactions for application in energetic materials. Cent. Eur. J. Energet. Mater. 2010, 7, 115–129. [Google Scholar]






| C wt.% | H wt.% | O wt.% | |
|---|---|---|---|
| Csuc | 85.7 | 3.2 | 11.1 |
| Ccell | 82.0 | 3.3 | 14.7 |
| Impact (J) | Friction (N) | |
|---|---|---|
| Standards | 2 | 80 |
| Al/WO3 | 42.2 | 8 |
| Al/WO3/Csuc | >49.6 | 216 |
| Al/WO3/Ccell | 47.1 | 324 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibot, P.; Miesch, Q.; Bach, A.; Schnell, F.; Gadiou, R.; Spitzer, D. Mechanical Desensitization of an Al/WO3 Nanothermite by Means of Carbonaceous Coatings Derived from Carbohydrates. C 2019, 5, 37. https://0-doi-org.brum.beds.ac.uk/10.3390/c5030037
Gibot P, Miesch Q, Bach A, Schnell F, Gadiou R, Spitzer D. Mechanical Desensitization of an Al/WO3 Nanothermite by Means of Carbonaceous Coatings Derived from Carbohydrates. C. 2019; 5(3):37. https://0-doi-org.brum.beds.ac.uk/10.3390/c5030037
Chicago/Turabian StyleGibot, Pierre, Quentin Miesch, Arnaud Bach, Fabien Schnell, Roger Gadiou, and Denis Spitzer. 2019. "Mechanical Desensitization of an Al/WO3 Nanothermite by Means of Carbonaceous Coatings Derived from Carbohydrates" C 5, no. 3: 37. https://0-doi-org.brum.beds.ac.uk/10.3390/c5030037