Graphene Nanoplatelets-Based Ni-Zeolite Composite Catalysts for Heptane Hydrocracking
Abstract
:1. Introduction
2. Materials and Method
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.2.1. BET Surface Area
2.2.2. Water Adsorption
2.2.3. X-ray Diffraction
2.2.4. Raman Spectroscopy
2.2.5. Scanning/Transmission Electron Microscopy Imaging (SEM/TEM)
2.2.6. Temperature-Programmed Desorption (TPD)
2.3. Catalytic Testing
3. Results and Discussion
3.1. Textural Studies
3.2. Water Adsorption Studies
3.3. Structural and Morphological Studies
3.4. Surface Acidity
3.5. Catalytic Performance
4. Conclusions
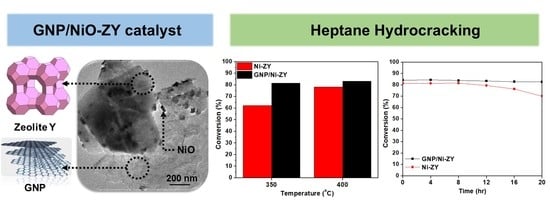
- GNPs are hydrophobic, and thus they add hydrophobic sites in the composite catalyst, which enhances the affinity between the catalyst and heptane during the reaction, improving its catalytic performance;
- As compared with the reduced NiO-ZY, the reduced GNP/NiO-ZY catalyst results in a 31% higher conversion percentage at 350 °C and 6% at 400 °C;
- The time-on-stream tests revealed that the reduced GNP/NiO-ZY had a significantly better stability with a drop in conversion of less than 2% after 20 h of time-on-stream as compared with the 14% drop in the reduced NiO-ZY;
- The reduced GNP/NiO-ZY catalyst favored the cracking into lighter molecules, as a high selectivity towards propane was reported. The reduced NiO-ZY, on the other hand, favored a high selectivity towards iso-butane and n-hexane.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nguyen, M.T.; Nguyen, N.T.; Cho, J.; Park, C.; Park, S.; Jung, J.; Lee, C.W. A review on the oil-soluble dispersed catalyst for slurry-phase hydrocracking of heavy oil. J. Ind. Eng. Chem. 2016, 43, 1–12. [Google Scholar] [CrossRef]
- Busca, G. Acidity and basicity of zeolites: A fundamental approach. Microporous Mesoporous Mater. 2017, 254, 3–16. [Google Scholar] [CrossRef]
- Dyer, A. Zeolites. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 9859–9863. [Google Scholar]
- Fatima, S.; Singaravel, G.; Hashaikeh, R. Ni-W/nano zeolite Y catalysts for n-heptane hydrocracking. Mater. Chem. Phys. 2018, 212, 87–94. [Google Scholar]
- Hashaikeh, R. Insight into ball milling for size reduction and nanoparticles production of H-Y zeolite. Mater. Chem. Phys. 2018, 220, 322–330. [Google Scholar] [CrossRef]
- Zhuman, B.; Anis, S.F.; Hashaikeh, R. Obtaining high crystalline ball milled H-Y zeolite particles with carbon nanostructures as a damping material. Microporous Mesoporous Mater. 2019, 273, 19–25. [Google Scholar] [CrossRef]
- Kazakov, M.O.; Nadeina, K.A.; Danilova, I.G.; Dik, P.P.; Klimov, O.V.; Pereyma, V.Y.; Paukshtis, E.A.; Golubev, I.S.; Prosvirin, I.P.; Gerasimov, E.Y.; et al. Influence of USY Zeolite Recrystallization on Physicochemical Properties and Catalytic Performance of NiMo/USY-Al2O3 Hydrocracking Catalysts. Catal. Today 2019, 329, 108–115. [Google Scholar] [CrossRef]
- Martens, J.A.; Verboekend, D.; Thomas, K.; Vanbutsele, G.; Pérez-Ramírez, J.; Gilson, J.P. Hydroisomerization and hydrocracking of linear and multibranched long model alkanes on hierarchical Pt/ZSM-22 zeolite. Catal. Today 2013, 218–219, 135–142. [Google Scholar] [CrossRef]
- Verheyen, E.; Jo, C.; Kurttepeli, M.; Vanbutsele, G.; Gobechiya, E.; Korányi, T.I.; Bals, S.; Van Tendeloo, G.; Ryoo, R.; Kirschhock, C.E.A.; et al. Molecular shape-selectivity of MFI zeolite nanosheets in n-decane isomerization and hydrocracking. J. Catal. 2013, 300, 70–80. [Google Scholar] [CrossRef]
- Kostoglou, N.; Lukovic, J.; Babic, B.; Matovic, B.; Photiou, D.; Constantinides, G.; Polychronopoulou, K.; Ryzhkov, V.; Grossmann, B.; Mitterer, C.; et al. Few-step synthesis, thermal purification and structural characterization of porous boron nitride nanoplatelets. Mater. Des. 2016, 110, 540–548. [Google Scholar] [CrossRef]
- Birch, M.E.; Ruda-Eberenz, T.A.; Chai, M.; Andrews, R.; Hatfield, R.L. Properties that Influence the Specific Surface Areas of Carbon Nanotubes and Nanofibers. Ann. Occup. Hyg. 2013, 57, 1148–1166. [Google Scholar]
- Zhang, L.; Zhang, F.; Yang, X.; Long, G.; Wu, Y.; Zhang, T.; Leng, K.; Huang, Y.; Ma, Y.; Yu, A.; et al. Porous 3D graphene-based bulk materials with exceptional high surface area and excellent conductivity for supercapacitors. Sci. Rep. 2013, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Reinoso, F. The Role of Carbon Materials in Heterogeneous Catalysis. Carbon N. Y. 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H.T. Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Soares, O.S.G.P. Development of carbon materials as metal catalyst supports and metal-free catalysts for catalytic reduction of ions and advanced oxidation processes. Bol. Grupo Esapnol Carbon 2016, 40, 20–23. [Google Scholar]
- Bandosz, T.J. Surface Chemistry of Carbon Materials. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 45–92. [Google Scholar]
- Wong, W.Y.; Daud, W.R.W.; Mohamad, A.B.; Kadhum, A.A.H.; Loh, K.S.; Majlan, E.H. Recent progress in nitrogen-doped carbon and its composites as electrocatalysts for fuel cell applications. Int. J. Hydrogen Energy 2013, 38, 9370–9386. [Google Scholar] [CrossRef]
- Boehm, H.-P. Catalytic Properties of Nitrogen-Containing Carbons. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 219–265. [Google Scholar]
- Ng, E.P.; Mintova, S. Nanoporous materials with enhanced hydrophilicity and high water sorption capacity. Microporous Mesoporous Mater. 2008, 114, 1–26. [Google Scholar] [CrossRef]
- Kitao, O.; Gubbins, K.E. Theoretical studies on VPI-5. 2. Energy decomposition analysis of the hydrophilicity. J. Phys. Chem. 1996, 100, 12424–12430. [Google Scholar] [CrossRef]
- Olson, D.H.; Haag, W.O.; Borghard, W.S. Use of water as a probe of zeolitic properties: Interaction of water with HZSM-5. Microporous Mesoporous Mater. 2000, 35–36, 435–446. [Google Scholar] [CrossRef]
- Gianotti, E.; Marchese, L.; Martra, G.; Coluccia, S. The interaction of NO with Co2+/Co3+ redox centres in CoAPOs catalysts: FTIR and UV-VIS investigations. Catal. Today 1999, 54, 547–552. [Google Scholar] [CrossRef]
- Afanassyev, I.S.; Moroz, N.K. Proton transfer in hydrated ammonium zeolites: A 1H NMR study of NH4-chabazite and NH4-clinoptilolite. Solid State Ion. 2003, 160, 125–129. [Google Scholar] [CrossRef]
- Koller, H.; Lobo, R.F.; Burkett, S.L.; Davis, M.E. SiO-...HOSi Hydrogen Bonds in As-Synthesized High-Silica Zeolites. J. Phys. Chem. 1995, 99, 12588–12596. [Google Scholar] [CrossRef]
- Oh, J.S.; Shim, W.G.; Lee, J.W.; Kim, J.H.; Moon, H.; Seo, G. Adsorption Equilibrium of Water Vapor on Mesoporous Materials. J. Chem. Eng. Data 2003, 48, 1458–1462. [Google Scholar] [CrossRef]
- Jing, M.; Wang, C.; Hou, H.; Wu, Z.; Zhu, Y.; Yang, Y.; Jia, X.; Zhang, Y.; Ji, X. Ultrafine nickel oxide quantum dots enbedded with few-layer exfoliative graphene for an asymmetric supercapacitor: Enhanced capacitances by alternating voltage. J. Power Sources 2015, 298, 241–248. [Google Scholar] [CrossRef]
- Gómez, M.J.; Loiácono, A.; Pérez, L.A.; Franceschini, E.A.; Lacconi, G.I. Highly Efficient Hybrid Ni/Nitrogenated Graphene Electrocatalysts for Hydrogen Evolution Reaction. ACS Omega 2019, 4, 2206–2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regulska, E.; Breczko, J.; Basa, A. Pristine and Graphene-Quantum-Dots-Decorated Spinel Nickel Aluminate for Water Remediation from Dyes and Toxic Pollutants. Water 2019, 11, 953. [Google Scholar] [CrossRef] [Green Version]
- Yasin, G.; Khan, M.A.; Arif, M.; Shakeel, M.; Hassan, T.M.; Khan, W.Q.; Korai, R.M.; Abbas, Z.; Zuo, Y. Synthesis of spheres-like Ni/graphene nanocomposite as an efficient anti-corrosive coating; effect of graphene content on its morphology and mechanical properties. J. Alloys Compd. 2018, 755, 79–88. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Tzitzios, V.; Sunny, K.; Polychronopoulou, K.; Basina, G.; Ismail, I.; Pillai, V.; Tharalekshmy, A.; Stephen, S.; Alhassan, S.M. Synthesis of nanoporous zeolite-Y and zeolite-Y/GO nanocomposite using polyelectrolyte functionalized graphene oxide. Surf. Coat. Technol. 2018, 350, 369–375. [Google Scholar] [CrossRef]
- Lin, K.Y.A.; Lee, W. Der Highly efficient removal of Malachite green from water by a magnetic reduced graphene oxide/zeolitic imidazolate framework self-assembled nanocomposite. Appl. Surf. Sci. 2016, 361, 114–121. [Google Scholar] [CrossRef]
- Khatamian, M.; Divband, B.; Farahmand-Zahed, F. Synthesis and characterization of Zinc (II)-loaded Zeolite/Graphene oxide nanocomposite as a new drug carrier. Mater. Sci. Eng. C 2016, 66, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Gong, P.; Li, B.; Kong, X.; Shakeel, M.; Liu, J.; Zuo, S. Hybriding hierarchical zeolite with Pt nanoparticles and graphene: Ternary nanocomposites for efficient visible-light photocatalytic degradation of methylene blue. Microporous Mesoporous Mater. 2018, 260, 180–189. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, Y.; Gao, Y.; Wu, J.; Hu, J.; Stein, A.; Tang, B. Nanocomposites of zeolitic imidazolate frameworks on graphene oxide for pseudocapacitor applications. J. Appl. Electrochem. 2016, 46, 441–450. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Papageridis, K.N.; Tzounis, L.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; AlKetbi, M.; Polychronopoulou, K.; Goula, M.A. Ni supported on CaO-MgO-Al2O3 as a highly selective and stable catalyst for H2 production via the glycerol steam reforming reaction. Int. J. Hydrogen Energy 2019, 44, 256–273. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Papageridis, K.N.; Siakavelas, G.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. The influence of SiO2 doping on the Ni/ZrO2 supported catalyst for hydrogen production through the glycerol steam reforming reaction. Catal. Today 2019, 319, 206–219. [Google Scholar] [CrossRef] [Green Version]
- Kanakia, S.; Toussaint, J.D.; Mullick Chowdhury, S.; Lalwani, G.; Tembulkar, T.; Button, T.; Shroyer, K.R.; Moore, W.; Sitharaman, B. Physicochemical characterization of a novel graphene-based magnetic resonance imaging contrast agent. Int. J. Nanomed. 2013, 8, 2821–2833. [Google Scholar]
- Cai, L.; Al-Ostaz, A.; Li, X.; Drzal, L.T.; Rook, B.P.; Cheng, A.H.D.; Alkhateb, H. Processing and mechanical properties investigation of epoxy-impregnated graphene paper. J. Nanomech. Micromech. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S. Characterization of electrical heating textile coated by graphene nanoplatelets/PVDF-HFP composite with various high graphene nanoplatelet contents. Polymers 2019, 11, 928. [Google Scholar] [CrossRef] [Green Version]
- Viculis, L.M.; Mack, J.J.; Mayer, O.M.; Hahn, H.T.; Kaner, R.B. Intercalation and exfoliation routes to graphite nanoplatelets. J. Mater. Chem. 2005, 15, 974–978. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Fagerlund, G. Determination of specific surface by the BET method. Matériaux Constr. 1973, 6, 239–245. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after Sixty Years: A Survey and Some New Results in the Determination of Crystallite Size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Grösse und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachr. Ges. Wiss. Göttingen 1918, 26, 98–100. [Google Scholar]
- Miskolczi, N.; Juzsakova, T.; Sója, J. Preparation and application of metal loaded ZSM-5 and y-zeolite catalysts for thermo-catalytic pyrolysis of real end of life vehicle plastics waste. J. Energy Inst. 2019, 92, 118–127. [Google Scholar] [CrossRef]
- Alothman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems With Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Chen, N.Y. Hydrophobic properties of zeolites. J. Phys. Chem. 1976, 80, 60–64. [Google Scholar] [CrossRef]
- Sharma, P.; Song, J.S.; Han, M.H.; Cho, C.H. GIS-NaP1 zeolite microspheres as potential water adsorption material: Influence of initial silica concentration on adsorptive and physical/topological properties. Sci. Rep. 2016, 6, 1–26. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Zhu, Y.; Che, J.; Xiao, Y. Two-dimensional transparent hydrophobic coating based on liquid-phase exfoliated graphene fluoride. Carbon N. Y. 2013, 63, 149–156. [Google Scholar] [CrossRef]
- Prolongo, S.G.; Moriche, R.; Jiménez-Suárez, A.; Sánchez, M.; Ureña, A. Advantages and disadvantages of the addition of graphene nanoplatelets to epoxy resins. Eur. Polym. J. 2014, 61, 206–214. [Google Scholar] [CrossRef]
- Richardson, J.T.; Scates, R.; Twigg, M.V. X-ray diffraction study of nickel oxide reduction by hydrogen. Appl. Catal. A Gen. 2003, 246, 137–150. [Google Scholar] [CrossRef]
- Vakili, M.; Khosrojerdi, S.; Aghajannezhad, P.; Yahyaei, M. A hybrid artificial neural network-genetic algorithm modeling approach for viscosity estimation of graphene nanoplatelets nanofluid using experimental data. Int. Commun. Heat Mass Transf. 2017, 82, 40–48. [Google Scholar] [CrossRef]
- Kim, H.N.; Suslick, K.S. The effects of ultrasound on crystals: Sonocrystallization and sonofragmentation. Crystals 2018, 8, 280. [Google Scholar] [CrossRef] [Green Version]
- Casimir, D.; Alghamdi, H.; Ahmed, I.Y.; Garcia-Sanchez, R.; Misra, P. Raman Spectroscopy of Graphene, Graphite and Graphene Nanoplatelets. In 2D Materials; IntechOpen: London, UK, 2019; p. 13. [Google Scholar]
- Kostoglou, N.; Tzitzios, V.; Kontos, A.G.; Giannakopoulos, K.; Tampaxis, C.; Papavasiliou, A.; Charalambopoulou, G.; Steriotis, T.; Li, Y.; Liao, K.; et al. Synthesis of nanoporous graphene oxide adsorbents by freeze-drying or microwave radiation: Characterization and hydrogen storage properties. Int. J. Hydrogen Energy 2015, 40, 6844–6852. [Google Scholar] [CrossRef]
- Gokus, T.; Nair, R.; Bonetti, A.; Bohmler, M.; Lombardo, A.; Novoselov, K.; Geim, A.; Ferrari, A.; Hartschuh, A. Making Graphene Luminescent by Oxygen Plasma Treatment. ACS Nano 2009, 3, 3963–3968. [Google Scholar] [CrossRef] [Green Version]
- Charisiou, N.D.; Siakavelas, G.I.; Dou, B.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. Nickel Supported on AlCeO3 as a Highly Selective and Stable Catalyst for Hydrogen Production via the Glycerol Steam Reforming Reaction. Catalysts 2019, 9, 411. [Google Scholar] [CrossRef] [Green Version]
- Miranda, B.C.; Chimentão, R.J.; Santos, J.B.O.; Gispert-Guirado, F.; Llorca, J.; Medina, F.; Bonillo, F.L.; Sueiras, J.E. Conversion of glycerol over 10%Ni/γ-Al2O3 catalyst. Appl. Catal. B Environ. 2014, 147, 464–480. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Iordanidis, A.; Polychronopoulou, K.; Yentekakis, I.V.; Goula, M.A. Studying the stability of Ni supported on modified with CeO2 alumina catalysts for the biogas dry reforming reaction. Mater. Today Proc. 2018, 5, 27607–27616. [Google Scholar] [CrossRef]
- Lin, T.J.; Meng, X.; Shi, L. Ni-exchanged Y-zeolite: An efficient heterogeneous catalyst for acetylene hydrocarboxylation. Appl. Catal. A Gen. 2014, 485, 163–171. [Google Scholar] [CrossRef]
- Polak, J.; Lu, B.C.-Y. Mutual Solubilities of Hydrocarbons and Water at 0 and 25 °C. Can. J. Chem. 1973, 51, 4018–4023. [Google Scholar] [CrossRef] [Green Version]







| BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | ||||
|---|---|---|---|---|---|---|
| Micropore | Mesopore | Total | Adsorption | Desorption | ||
| GNP | 300 | ̶ | ̶ | ̶ | ̶ | ̶ |
| ZY | 559 | 0.23 | 0.14 | 0.37 | 2.67 | 2.68 |
| NiO-ZY | 536 | 0.20 | 0.18 | 0.38 | 2.86 | 2.87 |
| GNP/NiO-ZY | 458 | 0.17 | 0.19 | 0.36 | 3.11 | 3.14 |
| Catalyst Composition | Crystallite Size, DNiO (nm) |
|---|---|
| NiO-ZY | 9.5 |
| GNP/NiO-ZY | 7.8 |
| GNP | GNP/NiO-ZY | |||
|---|---|---|---|---|
| Peak | Raman Shift (cm−1) | Area | Raman Shift (cm−1) | Area |
| D-band | 1340.6 | 1478.5 | 1341.3 | 1333.1 |
| G-band | 1579.0 | 1723.0 | 1583.6 | 1618.1 |
| G’-band | 2681.5 | 980.4 | 2693.0 | 770.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saab, R.; Polychronopoulou, K.; Charisiou, N.; Goula, M.A.; Schiffer, A. Graphene Nanoplatelets-Based Ni-Zeolite Composite Catalysts for Heptane Hydrocracking. C 2020, 6, 31. https://0-doi-org.brum.beds.ac.uk/10.3390/c6020031
Saab R, Polychronopoulou K, Charisiou N, Goula MA, Schiffer A. Graphene Nanoplatelets-Based Ni-Zeolite Composite Catalysts for Heptane Hydrocracking. C. 2020; 6(2):31. https://0-doi-org.brum.beds.ac.uk/10.3390/c6020031
Chicago/Turabian StyleSaab, Roba, Kyriaki Polychronopoulou, Nikolaos Charisiou, Maria A. Goula, and Andreas Schiffer. 2020. "Graphene Nanoplatelets-Based Ni-Zeolite Composite Catalysts for Heptane Hydrocracking" C 6, no. 2: 31. https://0-doi-org.brum.beds.ac.uk/10.3390/c6020031