Ultrasonication-Assisted Synthesis of ZnxCd1−xS for Enhanced Visible-Light Photocatalytic Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phase Structures and Morphology
2.2. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.3. Optical Property and Band Structure Analysis
2.4. Photocatalytic Activity and Stability
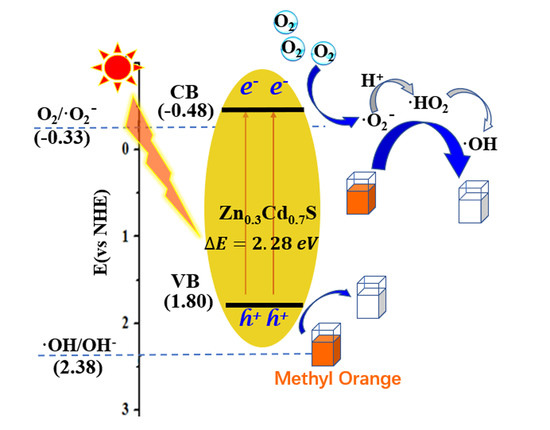
2.5. Mechanism on Enhancement in Photocatalytic Activity
3. Materials and Methods
3.1. Preparation of ZnxCd1−xS
3.2. Photocatalytic Tests
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Han, F.; Kambala, V.S.R.; Srinivasan, M.; Rajarathnam, D.; Naidu, R. Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review. Appl. Catal. A Gen. 2009, 359, 25–40. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.C.; Abdullah, A.Z.; Mohamed, A.R. Degradation of wastewaters containing organic dyes photocatalysed by zinc oxide: A review. Desalin. Water Treat. 2012, 41, 131–169. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Abdulkarem, A.M.; Aref, A.A.; Abdulhabeeb, A.; Li, Y.F.; Yu, Y. Synthesis of Bi2O3/Cu2O nanoflowers by hydrothermal method and its photocatalytic activity enhancement under simulated sunlight. J. Alloys Compd. 2013, 560, 132–141. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Yang, L.; Wang, Y.J.; Li, L.F.; Chen, S.F. High yield synthesis of homogeneous boron doping C3N4 nanocrystals with enhanced photocatalytic property. Appl. Surf. Sci. 2019, 489, 631–638. [Google Scholar] [CrossRef]
- Li, L.F.; Zhang, W.X.; Feng, C.; Luan, X.W.; Jiang, J.; Zhang, M.L. Preparation of nanocrystalline Cu2O by a modified solid-state reaction method and its photocatalytic activity. Mater. Lett. 2013, 107, 123–125. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Ye, Y.J.; Zhou, X.B.; Liu, Z.L.; Ma, D.; Li, B.; Liu, Q.H.; Zhu, G.P.; Chen, S.; Li, X. Facile preparation of a monodispersed CuO yolk-shelled structure with enhanced photochemical performance. Cryst. Eng. Commun. 2016, 18, 7994–8003. [Google Scholar] [CrossRef]
- Cheng, L.; Xiang, Q.J.; Liao, Y.L.; Zhang, H.W. CdS-Based photocatalysts. Environ. Sci. Technol. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Mort, J.; Spear, W.E. Hole drift mobility and lifetime in CdS crystals. Phys. Rev. Lett. 1962, 8, 314–315. [Google Scholar] [CrossRef]
- Deng, Q.; Miao, T.; Wang, Z.; Xu, Y.; Fu, X. Compositional regulation and modification of the host CdS for efficient photocatalytic hydrogen production: Case study on MoS2 decorated Co0.2Cd0.8S nanorods. Chem. Eng. J. 2019, 378, 122139. [Google Scholar] [CrossRef]
- Xie, Y.; Ali, G.; Yoo, S.H.; Cho, S.O. Sonication-assisted synthesis of CdS quantum-dot-sensitized TiO2 nanotube arrays with enhanced photoelectrochemical and photocatalytic activity. ACS Appl. Mater. Inter. 2010, 2, 2910–2914. [Google Scholar] [CrossRef]
- Fu, J.; Chang, B.B.; Tian, Y.L.; Xi, F.N.; Dong, X.P. Novel C3N4-CdS composite photocatalysts with organic-inorganic heterojunctions: In situ synthesis, exceptional activity, high stability and photocatalytic mechanism. J. Mater. Chem. A 2013, 1, 3083–3090. [Google Scholar] [CrossRef]
- Lin, Y.F.; Hsu, Y.J. Interfacial charge carrier dynamics of type-II semiconductor nanoheterostructures. Appl. Catal. B Environ. 2013, 130, 93–98. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, J.; Xiao, F.X.; Xiao, G. Revisiting the construction of graphene–CdS nanocomposites as efficient visible-light-driven photocatalysts for selective organic transformation. J. Mater. Chem. A 2014, 2, 5330–5339. [Google Scholar] [CrossRef]
- Fan, Q.J.; Huang, Y.N.; Zhang, C.; Liu, J.J.; Piao, L.Y.; Yu, Y.C.; Zuo, S.L.; Li, B.S. Superior nanoporous graphitic carbon nitride photocatalyst coupled with CdS quantum dots for photodegradation of RhB. Catal. Today 2016, 264, 250–256. [Google Scholar] [CrossRef]
- Tian, Q.; Wu, W.; Liu, J.; Wu, Z.; Yao, W.; Ding, J.; Jiang, C. Dimensional heterostructures of 1D CdS/2D ZnIn2S4 composited with 2D graphene: Designed synthesis and superior photocatalytic performance. Dalton Trans. 2017, 46, 2770–2777. [Google Scholar] [CrossRef]
- Lai, J.; Qin, Y.M.; Lan, Y.; Zhang, C. GSH-assisted hydrothermal synthesis of MnxCd1−xS solid solution hollow spheres and their application in photocatalytic degradation. Mat. Sci. Semicon. Proc. 2016, 52, 82–90. [Google Scholar] [CrossRef]
- Swafford, L.A.; Weigand, L.A.; Bowers, M.J.; McBride, J.R.; Rapaport, J.L.; Watt, T.L.; Dixit, S.K.; Feldman, L.C.; Rosenthal, S.J. Homogeneously alloyed CdSxSe1-x nanocrystals: Synthesis, characterization, and composition/size-dependent band gap. J. Am. Chem. Soc. 2006, 128, 12299–12306. [Google Scholar] [CrossRef]
- Kaur, M.; Nagaraja, C.M. Template-free synthesis of Zn1−xCdxS nanocrystals with tunable band structure for efficient water splitting and reduction of nitroaromatics in water. ACS Sustain. Chem. Eng. 2017, 5, 4293–4303. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, Z.; Hei, T.; Jiang, Y. One-pot synthesis of ZnxCd1−xS nanoparticles with nano-twin structure. J. Photochem. Photobiol. A 2019, 382, 11919. [Google Scholar] [CrossRef]
- Chen, J.; Lv, S.; Shen, Z.; Tian, P.; Chen, J.; Li, Y. Novel ZnCdS quantum dots engineering for enhanced visible-light-driven hydrogen evolution. ACS Sustain. Chem. Eng. 2019, 7, 13805–13814. [Google Scholar] [CrossRef]
- Tang, L.; Kuai, L.; Li, Y.; Li, H.; Zhou, Y.; Zou, Z. ZnxCd1−xS tunable band structure-directing photocatalytic activity and selectivity of visible-light reduction of CO2 into liquid solar fuels. Nanotechnology 2018, 29, 064003. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Wang, W.; Jia, X.; Wang, Y.; Zhou, S. A unique nanoporous graphene-ZnxCd1−xS hybrid nanocomposite for enhanced photocatalytic degradation of water pollutants. Ceram. Int. 2016, 42, 16775–16781. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.B.; Ren, Y.K.; Liu, E.Z.; Fan, J.; Hu, X.Y. Zn/Cd ratio-dependent synthetic conditions in ternary ZnCdS quantum dots. J. Alloys Compd. 2018, 752, 260–266. [Google Scholar] [CrossRef]
- Wang, X.; Tian, H.; Cui, X.; Zheng, W.; Liu, Y. One-pot hydrothermal synthesis of mesoporous ZnxCd1−xS/reduced graphene oxide hybrid material and its enhanced photocatalytic activity. Dalton Trans. 2014, 43, 12894–12903. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Xian, J.; Chen, W.; Hu, Y.; Shao, Y.; Fu, X. Specific analyses of the active species on Zn0.28Cd0.72S and TiO2 photocatalysts in the degradation of methyl orange. J. Phys. Chem. C 2010, 114, 21482–21492. [Google Scholar] [CrossRef]
- Li, W.J.; Li, D.Z.; Zhang, W.J.; Hu, Y.; He, Y.H.; Fu, X.Z. Microwave synthesis of ZnxCd1−xS nanorods and their photocatalytic activity under visible light. J. Phys. Chem. C 2010, 114, 2154–2159. [Google Scholar] [CrossRef]
- Abideen, Z.U.; Teng, F. Effect of alkaline treatment on photochemical activity and stability of Zn0.3Cd0.7S. Appl. Surf. Sci. 2019, 465, 459–469. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Moon, J.H.; Choi, Y.S.; Park, G.O.; Jin, M.; Jin, L.Y.; Li, D.; Lee, J.Y.; Son, S.U.; Kim, J.M. Visible-light driven photocatalytic degradation of organic dyes over ordered mesoporous CdxZn1–xS materials. J. Phys. Chem. C 2017, 121, 5137–5144. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, J.; Liu, J.; Shi, W.; Yang, G.; Wang, G.C.; Cheng, P. An efficient, visible-light-driven, hydrogen evolution catalyst NiS/ZnxCd1−xS nanocrystal derived from a metal-organic framework. Angew. Chem. Int. 2018, 57, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, G.; Li, Y.; Jin, Z. 2D/1D Zn0.7Cd0.3S p-n heterogeneous junction enhanced with NiWO4 for efficient photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2019, 554, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, D.; Chen, Z.; Huang, H.; Sun, M.; He, Y.; Fu, X. High-efficient degradation of dyes by ZnxCd1−xS solid solutions under visible light irradiation. J. Phys. Chem. C 2008, 112, 14943–14947. [Google Scholar] [CrossRef]
- Yu, J.; Yang, B.; Cheng, B. Noble-metal-free carbon nanotube-Cd0.1Zn0.9S composites for high visible-light photocatalytic H2-production performance. Nanoscale 2012, 4, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Meng, H.; Zhou, P.; Zheng, Y.; Wang, J.; Yu, J.; Gong, J. Zn1−xCdxS solid solutions with controlled bandgap and enhanced visible-light photocatalytic H2-production activity. ACS Catal. 2013, 3, 882–889. [Google Scholar] [CrossRef]
- Pugazhenthiran, N.; Sathishkumar, P.; Murugesan, S.; Anandan, S. Effective degradation of acid orange 10 by catalytic ozonation in the presence of Au-Bi2O3 nanoparticles. Chem. Eng. J. 2011, 168, 1227–1233. [Google Scholar] [CrossRef]
- Liao, K.H.; Lin, Y.S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Inter. 2011, 3, 2607–2615. [Google Scholar] [CrossRef]
- Ghows, N.; Entezari, M.H. Exceptional catalytic efficiency in mineralization of the reactive textile azo dye (RB5) by a combination of ultrasound and core-shell nanoparticles (CdS/TiO2). J. Hazard. Mater. 2011, 195, 132–138. [Google Scholar] [CrossRef]
- Zhong, J.; Zhang, Y.; Hu, C.; Hou, R.; Yin, H.; Li, H.; Huo, Y. Supercritical solvothermal preparation of a ZnxCd1−xS visible photocatalyst with enhanced activity. J. Mater. Chem. A 2014, 2, 19641–19647. [Google Scholar] [CrossRef]
- Zhong, X.; Feng, Y.; Knoll, W.; Han, M. Alloyed ZnxCd1−xS nanocrystals with highly narrow luminescence spectral width. J. Am. Chem. Soc. 2003, 125, 13559–13563. [Google Scholar] [CrossRef]
- Huang, M.; Yu, J.; Deng, C.; Huang, Y.; Fan, M.; Li, B.; Tong, Z.; Zhang, F.; Dong, L. 3D nanospherical CdxZn1-xS/reduced graphene oxide composites with superior photocatalytic activity and photocorrosion resistance. Appl. Surf. Sci. 2016, 365, 227–239. [Google Scholar] [CrossRef]
- Wu, Y.J.; Yue, Z.K.; Liu, A.J.; Yang, P.; Zhu, M.S. P-type Cu-doped Zn0.3Cd0.7S/Graphene photocathode for efficient water splitting in a photoelectrochemical tandem cell. ACS Sustain. Chem. Eng. 2016, 4, 2569–2577. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.R.; Ma, Y.L.; Jin, Z.L. Noble-metal-free visible light driven hetero-structural Ni/ZnxCd1−xS photocatalyst for efficient hydrogen production. Catal. Lett. 2019, 149, 1788–1799. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, J.; Jaroniec, M.; Gong, J.R. Noble metal-free reduced graphene oxide-ZnxCd1−xS nanocomposite with enhanced solar photocatalytic H2-production performance. Nano Lett. 2012, 12, 4584–4589. [Google Scholar] [CrossRef]
- Yi, S.S.; Yan, J.M.; Wulan, B.R.; Li, S.J.; Liu, K.H.; Jiang, Q. Noble-metal-free cobalt phosphide modified carbon nitride: An efficient photocatalyst for hydrogen generation. Appl. Catal. B Environ. 2017, 200, 477–483. [Google Scholar] [CrossRef]
- Wu, C.H.; Chang, H.W.; Chern, J.M. Basic dye decomposition kinetics in a photocatalytic slurry reactor. J. Hazard. Mater. 2006, 137, 336–343. [Google Scholar] [CrossRef]
- Wang, W.; Shen, H.; He, X.; Li, J. Effects of sulfur sources on properties of Cu2ZnSnS4 nanoparticles. J. Nanopart. Res. 2014, 16, 1–8. [Google Scholar] [CrossRef]








| Sample | Cd/Zn/S Molar Ratio | Size (nm) | Eg (eV) | d Value (Å) |
|---|---|---|---|---|
| CdS 0.1ZCS 0.2 ZCS 0.3 ZCS 0.4 ZCS 0.5 ZCS 0.6ZCS 0.7 ZCS 0.8 ZCS 0.9ZCS ZnS | Zn0Cd1.0S Zn0.1Cd0.9S Zn0.2Cd0.8S Zn0.3Cd0.7S Zn0.4Cd0.6S Zn0.5Cd0.5S Zn0.6Cd0.4S Zn0.7Cd0.3S Zn0.8Cd0.2S Zn0.9Cd0.1S Zn1.0Cd0S | 28.45 21.70 20.00 14.50 13.60 13.10 12.93 21.00 28.64 28.96 37.7 | 2.15 2.19 2.25 2.28 2.31 2.35 2.37 2.40 2.45 3.28 3.61 | 3.36 3.34 3.32 3.30 3.29 3.28 3.26 3.12 3.11 3.10 3.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhang, M.; Liu, M.; Fan, Y.; Ben, H.; Li, L.; Fu, X.; Chen, S. Ultrasonication-Assisted Synthesis of ZnxCd1−xS for Enhanced Visible-Light Photocatalytic Activity. Catalysts 2020, 10, 276. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10030276
Yang L, Zhang M, Liu M, Fan Y, Ben H, Li L, Fu X, Chen S. Ultrasonication-Assisted Synthesis of ZnxCd1−xS for Enhanced Visible-Light Photocatalytic Activity. Catalysts. 2020; 10(3):276. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10030276
Chicago/Turabian StyleYang, Lei, Maolin Zhang, Mingzhu Liu, You Fan, Haijie Ben, Longfeng Li, Xianliang Fu, and Shifu Chen. 2020. "Ultrasonication-Assisted Synthesis of ZnxCd1−xS for Enhanced Visible-Light Photocatalytic Activity" Catalysts 10, no. 3: 276. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10030276