Metal-Organic Frameworks as a Platform for CO2 Capture and Chemical Processes: Adsorption, Membrane Separation, Catalytic-Conversion, and Electrochemical Reduction of CO2
Abstract
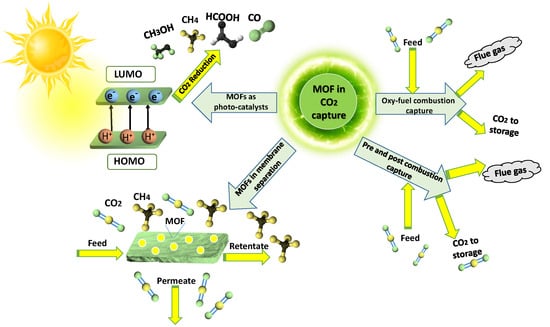
:1. Introduction
2. Fundamentals of Metal-Organic Frameworks (MOFs)
3. Carbon Dioxide (CO2) Capture Using Metal-Organic Frameworks
3.1. Oxy-Fuel Combustion CO2 Capture
3.2. Pre-Combustion CO2 Capture
3.3. Post-Combustion CO2 Capture
3.4. MOFs as Filler in Mixed-Matrix Membranes for CO2 Separation
3.5. MOFs in Photo-Catalytic Conversion of CO2
3.6. MOF-Based Materials for Electrochemical and Electrocatalytic Conversion of CO2
4. Conclusions and Future Perspective
Funding
Acknowledgments
Conflicts of Interest
References
- Smithson, P.A. IPCC, 2001: Climate Change 2001: The Scientific Basis. In Contribution of Working Group 1 to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Houghton, J.T., Ding, Y., Griggs, D.J., Noguer, M., van der Linden, P.J., Dai, X., Maskell, K., Johnson, C.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2001; Volume 22, p. 1144. ISBN 0-521-01495-6. [Google Scholar] [CrossRef]
- Lemke, P.; Ren, J.F.; Alley, R.; Allison, I.; Carrasco, J.; Flato, G.; Fujii, Y.; Kaser, G.; Mote, P.; Thomas, R.; et al. IPCC, 2007. Climate Change 2007. Synthesis Report. Contribution of Working Groups I, II & III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Thiruvenkatachari, R.; Su, S.; An, H.; Yu, X.X. Post combustion CO2 capture by carbon fibre monolithic adsorbents. Prog. Energy Combust. Sci. 2009, 35, 438–455. [Google Scholar] [CrossRef]
- Omoregbe, O.; Mustapha, A.N.; Steinberger-Wilckens, R.; El-Kharouf, A.; Onyeaka, H. Carbon capture technologies for climate change mitigation: A bibliometric analysis of the scientific discourse during 1998–2018. Energy Rep. 2020, 6, 1200–1212. [Google Scholar] [CrossRef]
- Ghoufi, A.; Maurin, G. Hybrid Monte Carlo Simulations Combined with a Phase Mixture Model to Predict the Structural Transitions of a Porous Metal−Organic Framework Material upon Adsorption of Guest Molecules. J. Phys. Chem. C 2010, 114, 6496–6502. [Google Scholar] [CrossRef]
- Younas, M.; Rezakazemi, M.; Daud, M.; Wazir, M.B.; Ahmad, S.; Ullah, N.; Inamuddin; Ramakrishna, S. Recent progress and remaining challenges in post-combustion CO2 capture using metal-organic frameworks (MOFs). Prog. Energy Combust. Sci. 2020, 80, 100849. [Google Scholar] [CrossRef]
- Qin, J.-S.; Yuan, S.; Alsalme, A.; Zhou, H.-C. Flexible Zirconium MOF as the Crystalline Sponge for Coordinative Alignment of Dicarboxylates. ACS Appl. Mater. Interfaces 2017, 9, 33408–33412. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Dybtsev, D.N.; Chun, H.; Yoon, S.H.; Kim, D.; Kim, K. Microporous Manganese Formate: A Simple Metal−Organic Porous Material with High Framework Stability and Highly Selective Gas Sorption Properties. J. Am. Chem. Soc. 2004, 126, 32–33. [Google Scholar] [CrossRef]
- Loiseau, T.; Lecroq, L.; Volkringer, C.; Marrot, J.; Férey, G.; Haouas, M.; Taulelle, F.; Bourrelly, S.; Llewellyn, P.L.; Latroche, M. MIL-96, a Porous Aluminum Trimesate 3D Structure Constructed from a Hexagonal Network of 18-Membered Rings and μ3-Oxo-Centered Trinuclear Units. J. Am. Chem. Soc. 2006, 128, 10223–10230. [Google Scholar] [CrossRef]
- Xue, M.; Ma, S.; Jin, Z.; Schaffino, R.M.; Zhu, G.-S.; Lobkovsky, E.B.; Qiu, S.-L.; Chen, B. Robust Metal−Organic Framework Enforced by Triple-Framework Interpenetration Exhibiting High H2 Storage Density. Inorg. Chem. 2008, 47, 6825–6828. [Google Scholar] [CrossRef]
- Zhuang, W.; Yuan, D.; Liu, D.; Zhong, C.; Li, J.-R.; Zhou, H.-C. Robust Metal–Organic Framework with An Octatopic Ligand for Gas Adsorption and Separation: Combined Characterization by Experiments and Molecular Simulation. Chem. Mater. 2012, 24, 18–25. [Google Scholar] [CrossRef]
- Scholes, C.; Kentish, S.; Stevens, G. Carbon Dioxide Separation through Polymeric Membrane Systems for Flue Gas Applications. Recent Pat. Chem. Eng. 2010, 1. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh Porosity in Metal-Organic Frameworks. Science 2010, 329, 424. [Google Scholar] [CrossRef] [Green Version]
- Qasem, N.A.A.; Ben-Mansour, R.; Habib, M.A. An efficient CO2 adsorptive storage using MOF-5 and MOF-177. Appl. Energy 2018, 210, 317–326. [Google Scholar] [CrossRef]
- Kayal, S.; Sun, B.; Chakraborty, A. Study of metal-organic framework MIL-101(Cr) for natural gas (methane) storage and compare with other MOFs (metal-organic frameworks). Energy 2015, 91, 772–781. [Google Scholar] [CrossRef]
- Wei, W.; Xia, Z.; Wei, Q.; Xie, G.; Chen, S.; Qiao, C.; Zhang, G.; Zhou, C. A heterometallic microporous MOF exhibiting high hydrogen uptake. Microporous Mesoporous Mater. 2013, 165, 20–26. [Google Scholar] [CrossRef]
- Joharian, M.; Morsali, A. Ultrasound-assisted synthesis of two new fluorinated metal-organic frameworks (F-MOFs) with the high surface area to improve the catalytic activity. J. Solid State Chem. 2019, 270, 135–146. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kumar, A.; Zaworotko, M.J. Metal-organic framework based carbon capture and purification technologies for clean environment. In Metal-Organic Frameworks (MOFs) for Environmental Applications; Ghosh, S.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 5–61. [Google Scholar] [CrossRef]
- Ghanbari, T.; Abnisa, F.; Wan Daud, W.M.A. A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci. Total Environ. 2020, 707, 135090. [Google Scholar] [CrossRef]
- Kazemi, S.; Safarifard, V. Carbon dioxide capture in MOFs: The effect of ligand functionalization. Polyhedron 2018, 154, 236–251. [Google Scholar] [CrossRef]
- Duan, C.; Yu, Y.; Xiao, J.; Li, Y.; Yang, P.; Hu, F.; Xi, H. Recent advancements in metal–organic frameworks for green applications. Green Energy Environ. 2020. [Google Scholar] [CrossRef]
- Simmons, J.M.; Wu, H.; Zhou, W.; Yildirim, T. Carbon capture in metal–organic frameworks—A comparative study. Energy Environ. Sci. 2011, 4, 2177–2185. [Google Scholar] [CrossRef]
- Li, J.-R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Koppens, F.H.L.; Folk, J.A.; Elzerman, J.M.; Hanson, R.; van Beveren, L.H.W.; Vink, I.T.; Tranitz, H.P.; Wegscheider, W.; Kouwenhoven, L.P.; Vandersypen, L.M.K. Control and Detection of Singlet-Triplet Mixing in a Random Nuclear Field. Science 2005, 309, 1346. [Google Scholar] [CrossRef] [Green Version]
- Ludig, S.; Haller, M.; Bauer, N. Tackling long-term climate change together: The case of flexible CCS and fluctuating renewable energy. Energy Procedia 2011, 4, 2580–2587. [Google Scholar] [CrossRef] [Green Version]
- Miyagawa, T.; Matsuhashi, R.; Murai, S.; Muraoka, M. Comparative assessment of CCS with other technologies mitigating climate change. Energy Procedia 2011, 4, 5710–5714. [Google Scholar] [CrossRef] [Green Version]
- Koljonen, T.; Flyktman, M.; Lehtilä, A.; Pahkala, K.; Peltola, E.; Savolainen, I. The role of CCS and renewables in tackling climate change. Energy Procedia 2009, 1, 4323–4330. [Google Scholar] [CrossRef] [Green Version]
- Freund, P. 1 - Anthropogenic climate change and the role of CO2 capture and storage (CCS). In Geological Storage of Carbon Dioxide (CO2); Gluyas, J., Mathias, S., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 3–25. [Google Scholar] [CrossRef]
- Hanaoka, T.; Masui, T. Exploring the 2 °C Target Scenarios by Considering Climate Benefits and Health Benefits—Role of Biomass and CCS. Energy Procedia 2017, 114, 2618–2630. [Google Scholar] [CrossRef]
- Gibbins, J.; Chalmers, H. Is all CCS equal? Classifying CCS applications by their potential climate benefit. Energy Procedia 2011, 4, 5715–5720. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhao, L.; Otto, A.; Robinius, M.; Stolten, D. A Review of Post-combustion CO2 Capture Technologies from Coal-fired Power Plants. Energy Procedia 2017, 114, 650–665. [Google Scholar] [CrossRef]
- Zou, X.; Zhu, G. CO2 Capture with MOF Membranes. In Microporous Materials for Separation Membranes; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 323–359. [Google Scholar] [CrossRef]
- Kang, Z.; Fan, L.; Sun, D. Recent advances and challenges of metal–organic framework membranes for gas separation. J. Mater. Chem. A 2017, 5, 10073–10091. [Google Scholar] [CrossRef]
- Chen, B.; Ma, S.; Hurtado, E.J.; Lobkovsky, E.B.; Zhou, H.-C. A Triply Interpenetrated Microporous Metal−Organic Framework for Selective Sorption of Gas Molecules. Inorg. Chem. 2007, 46, 8490–8492. [Google Scholar] [CrossRef]
- Llewellyn, P.L.; Bourrelly, S.; Serre, C.; Filinchuk, Y.; Férey, G. How hydration drastically improves adsorption selectivity for CO(2) over CH(4) in the flexible chromium terephthalate MIL-53. Angew. Chem. Int. Ed. Engl. 2006, 45, 7751–7754. [Google Scholar] [CrossRef]
- Chen, B.; Ma, S.; Zapata, F.; Fronczek, F.R.; Lobkovsky, E.B.; Zhou, H.-C. Rationally Designed Micropores within a Metal−Organic Framework for Selective Sorption of Gas Molecules. Inorg. Chem. 2007, 46, 1233–1236. [Google Scholar] [CrossRef]
- Cheon, Y.E.; Suh, M.P. Multifunctional Fourfold Interpenetrating Diamondoid Network: Gas Separation and Fabrication of Palladium Nanoparticles. Chem. A Eur. J. 2008, 14, 3961–3967. [Google Scholar] [CrossRef]
- Bourrelly, S.; Llewellyn, P.L.; Serre, C.; Millange, F.; Loiseau, T.; Férey, G. Different Adsorption Behaviors of Methane and Carbon Dioxide in the Isotypic Nanoporous Metal Terephthalates MIL-53 and MIL-47. J. Am. Chem. Soc. 2005, 127, 13519–13521. [Google Scholar] [CrossRef]
- Ma, S.; Wang, X.-S.; Manis, E.S.; Collier, C.D.; Zhou, H.-C. Metal−Organic Framework Based on a Trinickel Secondary Building Unit Exhibiting Gas-Sorption Hysteresis. Inorg. Chem. 2007, 46, 3432–3434. [Google Scholar] [CrossRef]
- Kitaura, R.; Seki, K.; Akiyama, G.; Kitagawa, S. Porous Coordination-Polymer Crystals with Gated Channels Specific for Supercritical Gases. Angew. Chem. Int. Ed. 2003, 42, 428–431. [Google Scholar] [CrossRef]
- Hayashi, H.; Côté, A.P.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. Zeolite A imidazolate frameworks. Nat. Mater. 2007, 6, 501–506. [Google Scholar] [CrossRef]
- Maji, T.K.; Matsuda, R.; Kitagawa, S. A flexible interpenetrating coordination framework with a bimodal porous functionality. Nat. Mater. 2007, 6, 142–148. [Google Scholar] [CrossRef]
- Thallapally, P.K.; Tian, J.; Radha Kishan, M.; Fernandez, C.A.; Dalgarno, S.J.; McGrail, P.B.; Warren, J.E.; Atwood, J.L. Flexible (breathing) interpenetrated metal-organic frameworks for CO2 separation applications. J. Am. Chem. Soc. 2008, 130, 16842–16843. [Google Scholar] [CrossRef]
- Maji, T.K.; Mostafa, G.; Matsuda, R.; Kitagawa, S. Guest-induced asymmetry in a metal-organic porous solid with reversible single-crystal-to-single-crystal structural transformation. J. Am. Chem. Soc. 2005, 127, 17152–17153. [Google Scholar] [CrossRef]
- Wade, C.R.; Dincă, M. Investigation of the synthesis, activation, and isosteric heats of CO2 adsorption of the isostructural series of metal–organic frameworks M3(BTC)2 (M = Cr, Fe, Ni, Cu, Mo, Ru). Dalton Trans. 2012, 41, 7931–7938. [Google Scholar] [CrossRef] [Green Version]
- Hong, D.H.; Suh, M.P. Selective CO2 adsorption in a metal–organic framework constructed from an organic ligand with flexible joints. Chem. Commun. 2012, 48, 9168–9170. [Google Scholar] [CrossRef]
- Bataille, T.; Bracco, S.; Comotti, A.; Costantino, F.; Guerri, A.; Ienco, A.; Marmottini, F. Solvent dependent synthesis of micro- and nano- crystalline phosphinate based 1D tubular MOF: Structure and CO2 adsorption selectivity. CrystEngComm 2012, 14, 7170–7173. [Google Scholar] [CrossRef]
- Yan, Q.; Lin, Y.; Wu, P.; Zhao, L.; Cao, L.; Peng, L.; Kong, C.; Chen, L. Designed Synthesis of Functionalized Two-Dimensional Metal–Organic Frameworks with Preferential CO2 Capture. ChemPlusChem 2013, 78, 86–91. [Google Scholar] [CrossRef]
- Li, T.; Sullivan, J.E.; Rosi, N.L. Design and Preparation of a Core–Shell Metal–Organic Framework for Selective CO2 Capture. J. Am. Chem. Soc. 2013, 135, 9984–9987. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Yun, R.; Bai, J.; Lu, Z.; Du, L.; Li, Y. Expanded porous MOF-505 analogue exhibiting large hydrogen storage capacity and selective carbon dioxide adsorption. Inorg. Chem. 2013, 52, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Li, J.-R.; Chen, Y.-P.; Yu, J.; Yakovenko, A.A.; Wang, Z.U.; Sun, L.-B.; Balbuena, P.B.; Zhou, H.-C. A versatile metal–organic framework for carbon dioxide capture and cooperative catalysis. Chem. Commun. 2012, 48, 9995–9997. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, H.; Zhong, Q. Synthesis and characterization of MOF-aminated graphite oxide composites for CO2 capture. Appl. Surf. Sci. 2013, 284, 138–144. [Google Scholar] [CrossRef]
- Qian, D.; Lei, C.; Hao, G.-P.; Li, W.-C.; Lu, A.-H. Synthesis of Hierarchical Porous Carbon Monoliths with Incorporated Metal–Organic Frameworks for Enhancing Volumetric Based CO2 Capture Capability. ACS Appl. Mater. Interfaces 2012, 4, 6125–6132. [Google Scholar] [CrossRef]
- Yu, J.; Balbuena, P.B. Water Effects on Postcombustion CO2 Capture in Mg-MOF-74. J. Phys. Chem. C 2013, 117, 3383–3388. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Stylianou, K.C.; Morsali, A.; Retailleau, P.; Maspoch, D. Selective CO2 Capture in Metal–Organic Frameworks with Azine-Functionalized Pores Generated by Mechanosynthesis. Cryst. Growth Des. 2014, 14, 2092–2096. [Google Scholar] [CrossRef]
- Lin, Y.; Yan, Q.; Kong, C.; Chen, L. Polyethyleneimine incorporated metal-organic frameworks adsorbent for highly selective CO2 capture. Sci. Rep. 2013, 3, 1859. [Google Scholar] [CrossRef]
- Caskey, S.R.; Wong-Foy, A.G.; Matzger, A.J. Dramatic Tuning of Carbon Dioxide Uptake via Metal Substitution in a Coordination Polymer with Cylindrical Pores. J. Am. Chem. Soc. 2008, 130, 10870–10871. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Peng, X.; Cheng, X.; Li, X.; Cao, D. CNT@Cu3(BTC)2 and Metal–Organic Frameworks for Separation of CO2/CH4 Mixture. J. Phys. Chem. C 2011, 115, 19864–19871. [Google Scholar] [CrossRef]
- Chen, S.; Jin, L.; Chen, X. The effect and prediction of temperature on adsorption capability of coal/CH4. Procedia Eng. 2011, 26, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, S.M.; Long, H.A. 13 - Integration of carbon capture in IGCC systems. In Integrated Gasification Combined Cycle (IGCC) Technologies; Wang, T., Stiegel, G., Eds.; Woodhead Publishing: Southorn, UK; Cambridge, UK, 2017; pp. 445–463. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Shah, B.B.; Zhao, D. CO2 Capture in Metal–Organic Framework Adsorbents: An Engineering Perspective. Adv. Sustain. Syst. 2019, 3, 1800080. [Google Scholar] [CrossRef] [Green Version]
- Günther, C.; Weng, M.; Kather, A. Restrictions and Limitations for the Design of a Steam Generator for a Coal-fired Oxyfuel Power Plant with Circulating Fluidised Bed Combustion. Energy Procedia 2013, 37, 1312–1321. [Google Scholar] [CrossRef] [Green Version]
- Jansen, D.; Gazzani, M.; Manzolini, G.; Dijk, E.v.; Carbo, M. Pre-combustion CO2 capture. Int. J. Greenh. Gas Control 2015, 40, 167–187. [Google Scholar] [CrossRef]
- Looyd, P.J.D. Precombustion technologies to aid carbon capture. In Greenhouse Gas Control Technologies 7; Rubin, E.S., Keith, D.W., Gilboy, C.F., Wilson, M., Morris, T., Gale, J., Thambimuthu, K., Eds.; Elsevier Science Ltd.: Oxford, UK, 2005; pp. 1957–1961. [Google Scholar] [CrossRef]
- Zhong, D.-L.; Wang, J.-L.; Lu, Y.-Y.; Li, Z.; Yan, J. Precombustion CO2 capture using a hybrid process of adsorption and gas hydrate formation. Energy 2016, 102, 621–629. [Google Scholar] [CrossRef]
- Lea-Langton, A.; Andrews, G. Pre-combustion Technologies. In Biomass Energy with Carbon Capture and Storage (BECCS): Unlocking Negative Emissions; Wiley: Hoboken, NJ, USA, 2018; pp. 67–91. [Google Scholar]
- Zhang, Z.; Yao, Z.-Z.; Xiang, S.; Chen, B. Perspective of microporous metal–organic frameworks for CO2 capture and separation. Energy Environ. Sci. 2014, 7, 2868–2899. [Google Scholar] [CrossRef]
- Chung, Y.G.; Gómez-Gualdrón, D.A.; Li, P.; Leperi, K.T.; Deria, P.; Zhang, H.; Vermeulen, N.A.; Stoddart, J.F.; You, F.; Hupp, J.T.; et al. In silico discovery of metal-organic frameworks for precombustion CO2 capture using a genetic algorithm. Sci. Adv. 2016, 2, e1600909. [Google Scholar] [CrossRef] [Green Version]
- Nandi, S.; De Luna, P.; Daff, T.D.; Rother, J.; Liu, M.; Buchanan, W.; Hawari, A.I.; Woo, T.K.; Vaidhyanathan, R. A single-ligand ultra-microporous MOF for precombustion CO2 capture and hydrogen purification. Sci. Adv. 2015, 1, e1500421. [Google Scholar] [CrossRef] [Green Version]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yaghi, O.M. Carbon capture and conversion using metal–organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef] [PubMed]
- Herm, Z.R.; Swisher, J.A.; Smit, B.; Krishna, R.; Long, J.R. Metal−Organic Frameworks as Adsorbents for Hydrogen Purification and Precombustion Carbon Dioxide Capture. J. Am. Chem. Soc. 2011, 133, 5664–5667. [Google Scholar] [CrossRef]
- Asgari, M.; Queen, W. Carbon Capture in Metal–Organic Frameworks; Wiley: Hoboken, NJ, USA, 2018; pp. 1–78. [Google Scholar]
- Favre, E. Membrane processes and postcombustion carbon dioxide capture: Challenges and prospects. Chem. Eng. J. 2011, 171, 782–793. [Google Scholar] [CrossRef]
- Tillman, D.A. Chapter Nine—The Development of Postcombustion Control Technology. In Coal-Fired Electricity and Emissions Control; Tillman, D.A., Ed.; Butterworth-Heinemann: Oxford, UK, 2018; pp. 237–276. [Google Scholar] [CrossRef]
- Zamarripa, M.A.; Eslick, J.C.; Matuszewski, M.S.; Miller, D.C. Multi-objective Optimization of Membrane-based CO2 Capture. In Computer Aided Chemical Engineering; Eden, M.R., Ierapetritou, M.G., Towler, G.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 44, pp. 1117–1122. [Google Scholar]
- Breeze, P. Chapter 7—Carbon Capture and Storage. In Coal-Fired Generation; Breeze, P., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 73–86. [Google Scholar] [CrossRef] [Green Version]
- Ghoshal, S.; Zeman, F. Carbon dioxide (CO2) capture and storage technology in the cement and concrete industry. In Developments and Innovation in Carbon Dioxide (CO2) Capture and Storage Technology; Maroto-Valer, M.M., Ed.; Woodhead Publishing: Cambridge, UK, 2010; Volume 1, pp. 469–491. [Google Scholar]
- Spigarelli, B.P.; Kawatra, S.K. Opportunities and challenges in carbon dioxide capture. J. CO2 Util. 2013, 1, 69–87. [Google Scholar] [CrossRef]
- Hu, Z.; Khurana, M.; Seah, Y.H.; Zhang, M.; Guo, Z.; Zhao, D. Ionized Zr-MOFs for highly efficient post-combustion CO2 capture. Chem. Eng. Sci. 2015, 124, 61–69. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The U.S. Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Martínez, F.; Sanz, R.; Orcajo, G.; Briones, D.; Yángüez, V. Amino-impregnated MOF materials for CO2 capture at post-combustion conditions. Chem. Eng. Sci. 2016, 142, 55–61. [Google Scholar] [CrossRef]
- Pai, K.N.; Baboolal, J.D.; Sharp, D.A.; Rajendran, A. Evaluation of diamine-appended metal-organic frameworks for post-combustion CO2 capture by vacuum swing adsorption. Sep. Purif. Technol. 2019, 211, 540–550. [Google Scholar] [CrossRef]
- Hedin, N.; Andersson, L.; Bergström, L.; Yan, J. Adsorbents for the post-combustion capture of CO2 using rapid temperature swing or vacuum swing adsorption. Appl. Energy 2013, 104, 418–433. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Maurya, M.; Singh, J.K. Effect of Ionic Liquid Impregnation in Highly Water-Stable Metal–Organic Frameworks, Covalent Organic Frameworks, and Carbon-Based Adsorbents for Post-combustion Flue Gas Treatment. Energy Fuels 2019, 33, 3421–3428. [Google Scholar] [CrossRef]
- Babarao, R.; Jiang, J.W. Cation Characterization and CO2 Capture in Li+-Exchanged Metal−Organic Frameworks: From First-Principles Modeling to Molecular Simulation. Ind. Eng. Chem. Res. 2011, 50, 62–68. [Google Scholar] [CrossRef]
- Park, J.; Suh, B.L.; Kim, J. Computational Design of a Photoresponsive Metal–Organic Framework for Post Combustion Carbon Capture. J. Phys. Chem. C 2020, 124, 13162–13167. [Google Scholar] [CrossRef]
- Wang, Q.; Bai, J.; Lu, Z.; Pan, Y.; You, X. Finely tuning MOFs towards high-performance post-combustion CO2 capture materials. Chem. Commun. 2016, 52, 443–452. [Google Scholar] [CrossRef]
- Marti, A. Metal-Organic Frameworks Materials for Post-Combustion CO 2 Capture; Wiley: Hoboken, NJ, USA, 2018; pp. 79–111. [Google Scholar]
- Hu, Y.; Verdegaal, W.M.; Yu, S.-H.; Jiang, H.-L. Alkylamine-Tethered Stable Metal–Organic Framework for CO2 Capture from Flue Gas. ChemSusChem 2014, 7, 734–737. [Google Scholar] [CrossRef]
- Siagian, U.W.R.; Raksajati, A.; Himma, N.F.; Khoiruddin, K.; Wenten, I.G. Membrane-based carbon capture technologies: Membrane gas separation vs. membrane contactor. J. Nat. Gas Sci. Eng. 2019, 67, 172–195. [Google Scholar] [CrossRef]
- Khalilpour, R.; Mumford, K.; Zhai, H.; Abbas, A.; Stevens, G.; Rubin, E.S. Membrane-based carbon capture from flue gas: A review. J. Clean. Prod. 2015, 103, 286–300. [Google Scholar] [CrossRef]
- Krishna, R.; van Baten, J.M. In silico screening of zeolite membranes for CO2 capture. J. Membr. Sci. 2010, 360, 323–333. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Zhao, L.; Dutta, P.; Winston Ho, W.S. New Pebax®/zeolite Y composite membranes for CO2 capture from flue gas. J. Membr. Sci. 2015, 495, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Chen, Y.; Wang, B.; Sun, C.; Chakraborty, S.; Ramasubramanian, K.; Dutta, P.K.; Ho, W.S.W. Multilayer polymer/zeolite Y composite membrane structure for CO2 capture from flue gas. J. Membr. Sci. 2016, 498, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Nothling, M.D.; Webley, P.A.; Jin, J.; Fu, Q.; Qiao, G.G. High-throughput CO2 capture using PIM-1@MOF based thin film composite membranes. Chem. Eng. J. 2020, 396, 125328. [Google Scholar] [CrossRef]
- Sun, J.; Li, Q.; Chen, G.; Duan, J.; Liu, G.; Jin, W. MOF-801 incorporated PEBA mixed-matrix composite membranes for CO2 capture. Sep. Purif. Technol. 2019, 217, 229–239. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Hou, L.; Yang, C.; Shen, H.; Yang, K.; Wang, Z. Metal-organic framework MOF-801/PIM-1 mixed-matrix membranes for enhanced CO2/N2 separation performance. Sep. Purif. Technol. 2020, 250, 117198. [Google Scholar] [CrossRef]
- Majumdar, S.; Tokay, B.; Martin-Gil, V.; Campbell, J.; Castro-Muñoz, R.; Ahmad, M.Z.; Fila, V. Mg-MOF-74/Polyvinyl acetate (PVAc) mixed matrix membranes for CO2 separation. Sep. Purif. Technol. 2020, 238, 116411. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Peters, T.A.; Konnertz, N.M.; Visser, T.; Téllez, C.; Coronas, J.; Fila, V.; de Vos, W.M.; Benes, N.E. High-pressure CO2/CH4 separation of Zr-MOFs based mixed matrix membranes. Sep. Purif. Technol. 2020, 230, 115858. [Google Scholar] [CrossRef]
- Chen, K.; Xu, K.; Xiang, L.; Dong, X.; Han, Y.; Wang, C.; Sun, L.-B.; Pan, Y. Enhanced CO2/CH4 separation performance of mixed-matrix membranes through dispersion of sorption-selective MOF nanocrystals. J. Membr. Sci. 2018, 563, 360–370. [Google Scholar] [CrossRef]
- Jiamjirangkul, P.; Inprasit, T.; Intasanta, V.; Pangon, A. Metal organic framework-integrated chitosan/poly(vinyl alcohol) (PVA) nanofibrous membrane hybrids from green process for selective CO2 capture and filtration. Chem. Eng. Sci. 2020, 221, 115650. [Google Scholar] [CrossRef]
- Lee, D.-J.; Li, Q.; Kim, H.; Lee, K. Preparation of Ni-MOF-74 membrane for CO2 separation by layer-by-layer seeding technique. Microporous Mesoporous Mater. 2012, 163, 169–177. [Google Scholar] [CrossRef]
- Anastasiou, S.; Bhoria, N.; Pokhrel, J.; Kumar Reddy, K.S.; Srinivasakannan, C.; Wang, K.; Karanikolos, G.N. Metal-organic framework/graphene oxide composite fillers in mixed-matrix membranes for CO2 separation. Mater. Chem. Phys. 2018, 212, 513–522. [Google Scholar] [CrossRef]
- Yin, H.; Alkaş, A.; Zhang, Y.; Zhang, Y.; Telfer, S.G. Mixed matrix membranes (MMMs) using an emerging metal-organic framework (MUF-15) for CO2 separation. J. Membr. Sci. 2020, 609, 118245. [Google Scholar] [CrossRef]
- Wu, W.; Li, Z.; Chen, Y.; Li, W. Polydopamine-Modified Metal–Organic Framework Membrane with Enhanced Selectivity for Carbon Capture. Environ. Sci. Technol. 2019, 53, 3764–3772. [Google Scholar] [CrossRef] [PubMed]
- Chernikova, V.; Shekhah, O.; Belmabkhout, Y.; Eddaoudi, M. Nanoporous Fluorinated Metal–Organic Framework-Based Membranes for CO2 Capture. ACS Appl. Nano Mater. 2020, 3, 6432–6439. [Google Scholar] [CrossRef]
- Cheng, Y.; Tavares, S.R.; Doherty, C.M.; Ying, Y.; Sarnello, E.; Maurin, G.; Hill, M.R.; Li, T.; Zhao, D. Enhanced Polymer Crystallinity in Mixed-Matrix Membranes Induced by Metal–Organic Framework Nanosheets for Efficient CO2 Capture. ACS Appl. Mater. Interfaces 2018, 10, 43095–43103. [Google Scholar] [CrossRef] [PubMed]
- Altintas, C.; Keskin, S. Molecular Simulations of MOF Membranes and Performance Predictions of MOF/Polymer Mixed Matrix Membranes for CO2/CH4 Separations. ACS Sustain. Chem. Eng. 2019, 7, 2739–2750. [Google Scholar] [CrossRef]
- Prasetya, N.; Ladewig, B.P. New Azo-DMOF-1 MOF as a Photoresponsive Low-Energy CO2 Adsorbent and Its Exceptional CO2/N2 Separation Performance in Mixed Matrix Membranes. ACS Appl. Mater. Interfaces 2018, 10, 34291–34301. [Google Scholar] [CrossRef]
- Benzaqui, M.; Pillai, R.S.; Sabetghadam, A.; Benoit, V.; Normand, P.; Marrot, J.; Menguy, N.; Montero, D.; Shepard, W.; Tissot, A.; et al. Revisiting the Aluminum Trimesate-Based MOF (MIL-96): From Structure Determination to the Processing of Mixed Matrix Membranes for CO2 Capture. Chem. Mater. 2017, 29, 10326–10338. [Google Scholar] [CrossRef] [Green Version]
- Maina, J.W.; Schütz, J.A.; Grundy, L.; Des Ligneris, E.; Yi, Z.; Kong, L.; Pozo-Gonzalo, C.; Ionescu, M.; Dumée, L.F. Inorganic Nanoparticles/Metal Organic Framework Hybrid Membrane Reactors for Efficient Photocatalytic Conversion of CO2. ACS Appl. Mater. Interfaces 2017, 9, 35010–35017. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ma, X.; Kasik, A.; Li, Z.; Lin, Y.S. Gas Separation Properties of Metal Organic Framework (MOF-5) Membranes. Ind. Eng. Chem. Res. 2013, 52, 1102–1108. [Google Scholar] [CrossRef]
- Hu, Z.; Kang, Z.; Qian, Y.; Peng, Y.; Wang, X.; Chi, C.; Zhao, D. Mixed Matrix Membranes Containing UiO-66(Hf)-(OH)2 Metal–Organic Framework Nanoparticles for Efficient H2/CO2 Separation. Ind. Eng. Chem. Res. 2016, 55, 7933–7940. [Google Scholar] [CrossRef]
- Fan, L.; Kang, Z.; Shen, Y.; Wang, S.; Zhao, H.; Sun, H.; Hu, X.; Sun, H.; Wang, R.; Sun, D. Mixed Matrix Membranes Based on Metal–Organic Frameworks with Tunable Pore Size for CO2 Separation. Cryst. Growth Des. 2018, 18, 4365–4371. [Google Scholar] [CrossRef]
- Takht Ravanchi, M.; Sahebdelfar, S. Catalytic conversions of CO2 to help mitigate climate change: Recent process developments. Process Saf. Environ. Prot. 2021, 145, 172–194. [Google Scholar] [CrossRef]
- Dewangan, N.; Hui, W.M.; Jayaprakash, S.; Bawah, A.-R.; Poerjoto, A.J.; Jie, T.; Jangam, A.; Hidajat, K.; Kawi, S. Recent progress on layered double hydroxide (LDH) derived metal-based catalysts for CO2 conversion to valuable chemicals. Catal. Today 2020. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Catalytic thermal conversion of CO2 into fuels: Perspective and challenges. Renew. Sustain. Energy Rev. 2019, 115, 109333. [Google Scholar] [CrossRef]
- Yadav, D.K.; Singh, D.K.; Ganesan, V. Recent strategy(ies) for the electrocatalytic reduction of CO2: Ni single-atom catalysts for the selective electrochemical formation of CO in aqueous electrolytes. Curr. Opin. Electrochem. 2020, 22, 87–93. [Google Scholar] [CrossRef]
- Guo, W.; Shim, K.; Odongo Ngome, F.O.; Moon, Y.H.; Choi, S.-Y.; Kim, Y.-T. Highly active coral-like porous silver for electrochemical reduction of CO2 to CO. Appl. Surf. Sci. 2020, 41, 101242. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, T.; Liang, X.; Deng, X.; Guo, W.; Wang, Z.; Lu, X.; Wu, C.-M.L. Single transition metal atoms on nitrogen-doped carbon for CO2 electrocatalytic reduction: CO production or further CO reduction? Appl. Surf. Sci. 2020, 533, 147466. [Google Scholar] [CrossRef]
- Guo, W.; Shim, K.; Kim, Y.-T. Ag layer deposited on Zn by physical vapor deposition with enhanced CO selectivity for electrochemical CO2 reduction. Appl. Surf. Sci. 2020, 526, 146651. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Nawawi, M.G.M. Highly stable 3D/2D WO3/g-C3N4 Z-scheme heterojunction for stimulating photocatalytic CO2 reduction by H2O/H2 to CO and CH4 under visible light. J. CO2 Util. 2020, 41, 101270. [Google Scholar] [CrossRef]
- Kazemi Movahed, S.; Najinasab, A.; Nikbakht, R.; Dabiri, M. Visible light assisted photocatalytic reduction of CO2 to methanol using Fe3O4@N-C/Cu2O nanostructure photocatalyst. J. Photochem. Photobiol. A Chem. 2020, 401, 112763. [Google Scholar] [CrossRef]
- Jiang, X.X.; De Hu, X.; Tarek, M.; Saravanan, P.; Alqadhi, R.; Chin, S.Y.; Rahman Khan, M.M. Tailoring the properties of g-C3N4 with CuO for enhanced photoelectrocatalytic CO2 reduction to methanol. J. CO2 Util. 2020, 40, 101222. [Google Scholar] [CrossRef]
- Aranda-Aguirre, A.; Ojeda, J.; Ferreira de Brito, J.; Garcia-Segura, S.; Boldrin Zanoni, M.V.; Alarcon, H. Photoelectrodes of Cu2O with interfacial structure of topological insulator Bi2Se3 contributes to selective photoelectrocatalytic reduction of CO2 towards methanol. J. CO2 Util. 2020, 39, 101154. [Google Scholar] [CrossRef]
- Karim, K.M.R.; Tarek, M.; Sarkar, S.M.; Mouras, R.; Ong, H.R.; Abdullah, H.; Cheng, C.K.; Khan, M.M.R. Photoelectrocatalytic reduction of CO2 to methanol over CuFe2O4@PANI photocathode. Int. J. Hydrog. Energy 2020. [Google Scholar] [CrossRef]
- Huang, W.; Yuan, G. A composite heterogeneous catalyst C-Py-Sn-Zn for selective electrochemical reduction of CO2 to methanol. Electrochem. Commun. 2020, 118, 106789. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, X.; Xuan, X.; Liu, N.; Zhou, J. Development of an efficient catalyst with controlled sulfur vacancies and high pyridine nitrogen content for the photoelectrochemical reduction of CO2 into methanol. Sci. Total Environ. 2020, 702, 134981. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Zhang, X.; Ji, X. Recent progress on electrochemical reduction of CO2 to methanol. Curr. Opin. Green Sustain. Chem. 2020, 23, 10–17. [Google Scholar] [CrossRef]
- Tarek, M.; Rezaul Karim, K.M.; Sarkar, S.M.; Deb, A.; Ong, H.R.; Abdullah, H.; Cheng, C.K.; Rahman Khan, M.M. Hetero-structure CdS–CuFe2O4 as an efficient visible light active photocatalyst for photoelectrochemical reduction of CO2 to methanol. Int. J. Hydrogen Energy 2019, 44, 26271–26284. [Google Scholar] [CrossRef]
- Feng, S.; Zhao, J.; Bai, Y.; Liang, X.; Wang, T.; Wang, C. Facile synthesis of Mo-doped TiO2 for selective photocatalytic CO2 reduction to methane: Promoted H2O dissociation by Mo doping. J. CO2 Util. 2020, 38, 1–9. [Google Scholar] [CrossRef]
- Merino-Garcia, I.; Albo, J.; Solla-Gullón, J.; Montiel, V.; Irabien, A. Cu oxide/ZnO-based surfaces for a selective ethylene production from gas-phase CO2 electroconversion. J. CO2 Util. 2019, 31, 135–142. [Google Scholar] [CrossRef]
- Qin, T.; Qian, Y.; Zhang, F.; Lin, B.-L. Cloride-derived copper electrode for efficient electrochemical reduction of CO2 to ethylene. Chin. Chem. Lett. 2019, 30, 314–318. [Google Scholar] [CrossRef]
- Fan, L.; Xia, C.; Zhu, P.; Lu, Y.; Wang, H. Electrochemical CO2 reduction to high-concentration pure formic acid solutions in an all-solid-state reactor. Nat. Commun. 2020, 11, 3633. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, T.; Wang, Y.; Hao, R.; Wang, H.; Han, Y. Catalytic reduction of CO2 to HCO2− by nanoscale nickel-based bimetallic alloy under atmospheric pressure. J. Ind. Eng. Chem. 2019, 77, 291–302. [Google Scholar] [CrossRef]
- Li, P.; Liu, L.; An, W.; Wang, H.; Guo, H.; Liang, Y.; Cui, W. Ultrathin porous g-C3N4 nanosheets modified with AuCu alloy nanoparticles and C-C coupling photothermal catalytic reduction of CO2 to ethanol. Appl. Catal. B Environ. 2020, 266, 118618. [Google Scholar] [CrossRef]
- Tekalgne, M.A.; Do, H.H.; Hasani, A.; Van Le, Q.; Jang, H.W.; Ahn, S.H.; Kim, S.Y. Two-dimensional materials and metal-organic frameworks for the CO2 reduction reaction. Mater. Today Adv. 2020, 5, 100038. [Google Scholar] [CrossRef]
- Billo, T.; Shown, I.; Anbalagan, A.k.; Effendi, T.A.; Sabbah, A.; Fu, F.-Y.; Chu, C.-M.; Woon, W.-Y.; Chen, R.-S.; Lee, C.-H.; et al. A mechanistic study of molecular CO2 interaction and adsorption on carbon implanted SnS2 thin film for photocatalytic CO2 reduction activity. Nano Energy 2020, 72, 104717. [Google Scholar] [CrossRef]
- Mu, Q.; Zhu, W.; Li, X.; Zhang, C.; Su, Y.; Lian, Y.; Qi, P.; Deng, Z.; Zhang, D.; Wang, S.; et al. Electrostatic charge transfer for boosting the photocatalytic CO2 reduction on metal centers of 2D MOF/rGO heterostructure. Appl. Catal. B Environ. 2020, 262, 118144. [Google Scholar] [CrossRef]
- Ješić, D.; Lašič Jurković, D.; Pohar, A.; Suhadolnik, L.; Likozar, B. Engineering Photocatalytic and Photoelectrocatalytic CO2 Reduction Reactions: Mechanisms, Intrinsic Kinetics, Mass Transfer Resistances, Reactors and Multi-scale Modelling Simulations. Chem. Eng. J. 2020, 126799. [Google Scholar] [CrossRef]
- Omar, S.; Shkir, M.; Ajmal Khan, M.; Ahmad, Z.; AlFaify, S. A comprehensive study on molecular geometry, optical, HOMO-LUMO, and nonlinear properties of 1,3-diphenyl-2-propen-1-ones chalcone and its derivatives for optoelectronic applications: A computational approach. Optik 2020, 204, 164172. [Google Scholar] [CrossRef]
- Reddy, C.V.; Reddy, K.R.; Harish, V.V.N.; Shim, J.; Shankar, M.V.; Shetti, N.P.; Aminabhavi, T.M. Metal-organic frameworks (MOFs)-based efficient heterogeneous photocatalysts: Synthesis, properties and its applications in photocatalytic hydrogen generation, CO2 reduction and photodegradation of organic dyes. Int. J. Hydrog. Energy 2020, 45, 7656–7679. [Google Scholar] [CrossRef]
- Wang, C.-C.; Zhang, Y.-Q.; Li, J.; Wang, P. Photocatalytic CO2 reduction in metal–organic frameworks: A mini review. J. Mol. Struct. 2015, 1083, 127–136. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Z.; deKrafft, K.E.; Lin, W. Doping Metal–Organic Frameworks for Water Oxidation, Carbon Dioxide Reduction, and Organic Photocatalysis. J. Am. Chem. Soc. 2011, 133, 13445–13454. [Google Scholar] [CrossRef]
- Liu, Q.; Low, Z.-X.; Li, L.; Razmjou, A.; Wang, K.; Yao, J.; Wang, H. ZIF-8/Zn2GeO4 nanorods with an enhanced CO2 adsorption property in an aqueous medium for photocatalytic synthesis of liquid fuel. J. Mater. Chem. A 2013, 1, 11563–11569. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z. An Amine-Functionalized Titanium Metal–Organic Framework Photocatalyst with Visible-Light-Induced Activity for CO2 Reduction. Angew. Chem. Int. Ed. 2012, 51, 3364–3367. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hu, J.; Deng, M.; Wang, H.; Wang, X.; Hu, Y.; Jiang, H.L.; Jiang, J.; Zhang, Q.; Xie, Y.; et al. Integration of an inorganic semiconductor with a metal-organic framework: A platform for enhanced gaseous photocatalytic reactions. Adv. Mater. 2014, 26, 4783–4788. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Sun, Q.; Wang, Z.; Huang, B.; Dai, Y.; Qin, X.; Zhang, X. Chemical Adsorption Enhanced CO2 Capture and Photoreduction over a Copper Porphyrin Based Metal Organic Framework. ACS Appl. Mater. Interfaces 2013, 5, 7654–7658. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Liu, W.; Fu, Y.; Fang, Z.; Sun, F.; Fu, X.; Zhang, Y.; Li, Z. Noble Metals Can Have Different Effects on Photocatalysis Over Metal–Organic Frameworks (MOFs): A Case Study on M/NH2-MIL-125(Ti) (M=Pt and Au). Chem. A Eur. J. 2014, 20, 4780–4788. [Google Scholar] [CrossRef]
- Sun, D.; Liu, W.; Qiu, M.; Zhang, Y.; Li, Z. Introduction of a mediator for enhancing photocatalytic performance via post-synthetic metal exchange in metal–organic frameworks (MOFs). Chem. Commun. 2015, 51, 2056–2059. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, S.; Fei, H.; Kang, J.K.; Cohen, S.M. Photocatalytic CO2 reduction using visible light by metal-monocatecholato species in a metal–organic framework. Chem. Commun. 2015, 51, 16549–16552. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, X. Photocatalytic CO2 reduction by CdS promoted with a zeolitic imidazolate framework. Appl. Catal. B Environ. 2015, 162, 494–500. [Google Scholar] [CrossRef]
- Wang, M.; Wang, D.; Li, Z. Self-assembly of CPO-27-Mg/TiO2 nanocomposite with enhanced performance for photocatalytic CO2 reduction. Appl. Catal. B Environ. 2016, 183, 47–52. [Google Scholar] [CrossRef]
- Yan, S.; Ouyang, S.; Xu, H.; Zhao, M.; Zhang, X.; Ye, J. Co-ZIF-9/TiO2 nanostructure for superior CO2 photoreduction activity. J. Mater. Chem. A 2016, 4, 15126–15133. [Google Scholar] [CrossRef]
- Sadeghi, N.; Sharifnia, S.; Sheikh Arabi, M. A porphyrin-based metal organic framework for high rate photoreduction of CO2 to CH4 in gas phase. J. CO2 Util. 2016, 16, 450–457. [Google Scholar] [CrossRef]
- Xu, H.-Q.; Hu, J.; Wang, D.; Li, Z.; Zhang, Q.; Luo, Y.; Yu, S.-H.; Jiang, H.-L. Visible-Light Photoreduction of CO2 in a Metal–Organic Framework: Boosting Electron–Hole Separation via Electron Trap States. J. Am. Chem. Soc. 2015, 137, 13440–13443. [Google Scholar] [CrossRef]
- Goyal, S.; Shaharun, M.S.; Kait, C.F.; Abdullah, B.; Ameen, M. Photoreduction of Carbon Dioxide to Methanol over Copper Based Zeolitic Imidazolate Framework-8: A New Generation Photocatalyst. Catalysts 2018, 8, 581. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Han, L.; Zhou, J.; Sun, C.; Hu, C.; Wang, X.; Su, Z. Photoreduction of carbon dioxide under visible light by ultra-small Ag nanoparticles doped into Co-ZIF-9. Nanotechnology 2018, 29, 284003. [Google Scholar] [CrossRef]
- Han, B.; Ou, X.; Deng, Z.; Song, Y.; Tian, C.; Deng, H.; Xu, Y.-J.; Lin, Z. Nickel Metal–Organic Framework Monolayers for Photoreduction of Diluted CO2: Metal-Node-Dependent Activity and Selectivity. Angew. Chem. Int. Ed. 2018, 57, 16811–16815. [Google Scholar] [CrossRef]
- Chen, C.; Wu, T.; Wu, H.; Liu, H.; Qian, Q.; Liu, Z.; Yang, G.; Han, B. Highly effective photoreduction of CO2 to CO promoted by integration of CdS with molecular redox catalysts through metal–organic frameworks. Chem. Sci. 2018, 9, 8890–8894. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, N.; Sharifnia, S.; Do, T.-O. Enhanced CO2 photoreduction by a graphene–porphyrin metal–organic framework under visible light irradiation. J. Mater. Chem. A 2018, 6, 18031–18035. [Google Scholar] [CrossRef]
- Ye, L.; Gao, Y.; Cao, S.; Chen, H.; Yao, Y.; Hou, J.; Sun, L. Assembly of highly efficient photocatalytic CO2 conversion systems with ultrathin two-dimensional metal–organic framework nanosheets. Appl. Catal. B Environ. 2018, 227, 54–60. [Google Scholar] [CrossRef]
- Li, D.; Kassymova, M.; Cai, X.; Zang, S.-Q.; Jiang, H.-L. Photocatalytic CO2 reduction over metal-organic framework-based materials. Coord. Chem. Rev. 2020, 412, 213262. [Google Scholar] [CrossRef]
- Li, P.-Z.; Wang, X.-J.; Liu, J.; Phang, H.S.; Li, Y.; Zhao, Y. Highly Effective Carbon Fixation via Catalytic Conversion of CO2 by an Acylamide-Containing Metal–Organic Framework. Chem. Mater. 2017, 29, 9256–9261. [Google Scholar] [CrossRef]
- Ding, D.; Jiang, Z.; Jin, J.; Li, J.; Ji, D.; Zhang, Y.; Zan, L. Impregnation of semiconductor CdS NPs in MOFs cavities via double solvent method for effective photocatalytic CO2 conversion. J. Catal. 2019, 375, 21–31. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Q.-L. MOF-based materials for photo- and electrocatalytic CO2 reduction. EnergyChem 2020, 2, 100033. [Google Scholar] [CrossRef]
- Wang, L.; Jin, P.; Duan, S.; She, H.; Huang, J.; Wang, Q. In-situ incorporation of Copper(II) porphyrin functionalized zirconium MOF and TiO2 for efficient photocatalytic CO2 reduction. Sci. Bull. 2019, 64, 926–933. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Zhang, X.; Lu, Y.; Yang, Y.; Zhang, Y.-P.; Tang, H.-L.; Zhang, F.-M.; Yang, Z.-D.; Sun, X.; Feng, Y. Regulation of metal ions in smart metal-cluster nodes of metal-organic frameworks with open metal sites for improved photocatalytic CO2 reduction reaction. Appl. Catal. B Environ. 2020, 276, 119173. [Google Scholar] [CrossRef]
- Chen, S.; Yu, J.; Zhang, J. Enhanced photocatalytic CO2 reduction activity of MOF-derived ZnO/NiO porous hollow spheres. J. CO2 Util. 2018, 24, 548–554. [Google Scholar] [CrossRef]
- Crake, A.; Christoforidis, K.C.; Kafizas, A.; Zafeiratos, S.; Petit, C. CO2 capture and photocatalytic reduction using bifunctional TiO2/MOF nanocomposites under UV–vis irradiation. Appl. Catal. B Environ. 2017, 210, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Wu, D.; Han, X.; Wang, H.; Zhang, L.; Gao, Z.; Xu, F.; Jiang, K. Ultrasonic assisted synthesis of Zn-Ni bi-metal MOFs for interconnected Ni-N-C materials with enhanced electrochemical reduction of CO2. J. CO2 Util. 2019, 32, 251–258. [Google Scholar] [CrossRef]
- Kang, X.; Gollan, R.J.; Jacobs, P.A.; Veeraragavan, A. Suppression of instabilities in a premixed methane–air flame in a narrow channel via hydrogen/carbon monoxide addition. Combust. Flame 2016, 173, 266–275. [Google Scholar] [CrossRef]
- Wang, Y.-R.; Huang, Q.; He, C.-T.; Chen, Y.; Liu, J.; Shen, F.-C.; Lan, Y.-Q. Oriented electron transmission in polyoxometalate-metalloporphyrin organic framework for highly selective electroreduction of CO2. Nat. Commun. 2018, 9, 4466. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Liu, J.; Gao, Y.; Gong, C.; Addicoat, M.; Heine, T.; Wöll, C.; Sun, L. Highly oriented MOF thin film-based electrocatalytic device for the reduction of CO2 to CO exhibiting high faradaic efficiency. J. Mater. Chem. A 2016, 4, 15320–15326. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, P.; Wang, Z.; Kang, P. Zinc Imidazolate Metal–Organic Frameworks (ZIF-8) for Electrochemical Reduction of CO2 to CO. ChemPhysChem 2017, 18, 3142–3147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Yang, H.; Zhou, X.; Liu, K.; Zhang, C.; Zhou, Z.; Wang, C.; Lin, W. Electrocatalytic reduction of CO2 to CO with 100% faradaic efficiency by using pyrolyzed zeolitic imidazolate frameworks supported on carbon nanotube networks. J. Mater. Chem. A 2017, 5, 24867–24873. [Google Scholar] [CrossRef]
- Kornienko, N.; Zhao, Y.; Kley, C.S.; Zhu, C.; Kim, D.; Lin, S.; Chang, C.J.; Yaghi, O.M.; Yang, P. Metal–Organic Frameworks for Electrocatalytic Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2015, 137, 14129–14135. [Google Scholar] [CrossRef] [Green Version]
- Hinogami, R.; Yotsuhashi, S.; Deguchi, M.; Zenitani, Y.; Hashiba, H.; Yamada, Y. Electrochemical Reduction of Carbon Dioxide Using a Copper Rubeanate Metal Organic Framework. ECS Electrochem. Lett. 2012, 1, H17–H19. [Google Scholar] [CrossRef]
- Perfecto-Irigaray, M.; Albo, J.; Beobide, G.; Castillo, O.; Irabien, A.; Pérez-Yáñez, S. Synthesis of heterometallic metal–organic frameworks and their performance as electrocatalyst for CO2 reduction. RSC Adv. 2018, 8, 21092–21099. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Cai, F.; Li, H.; Wu, H.; Wang, G.; Li, Y.; Miao, S.; Xie, S.; Si, R.; Wang, J.; et al. Surface functionalization of ZIF-8 with ammonium ferric citrate toward high exposure of Fe-N active sites for efficient oxygen and carbon dioxide electroreduction. Nano Energy 2017, 38, 281–289. [Google Scholar] [CrossRef]
- Huan, T.N.; Ranjbar, N.; Rousse, G.; Sougrati, M.; Zitolo, A.; Mougel, V.; Jaouen, F.; Fontecave, M. Electrochemical Reduction of CO2 Catalyzed by Fe-N-C Materials: A Structure–Selectivity Study. ACS Catal. 2017, 7, 1520–1525. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Yu, C.; Zhao, C.; Huang, H.; Yao, X.; Han, X.; Guo, W.; Cui, S.; Huang, H.; Qiu, J. Restructuring of Cu2O to Cu2O@Cu-Metal–Organic Frameworks for Selective Electrochemical Reduction of CO2. ACS Appl. Mater. Interfaces 2019, 11, 9904–9910. [Google Scholar] [CrossRef]
- Li, F.; Gu, G.H.; Choi, C.; Kolla, P.; Hong, S.; Wu, T.-S.; Soo, Y.-L.; Masa, J.; Mukerjee, S.; Jung, Y.; et al. Highly stable two-dimensional bismuth metal-organic frameworks for efficient electrochemical reduction of CO2. Appl. Catal. B Environ. 2020, 277, 119241. [Google Scholar] [CrossRef]
- Rayer, A.V.; Reid, E.; Kataria, A.; Luz, I.; Thompson, S.J.; Lail, M.; Zhou, J.; Soukri, M. Electrochemical carbon dioxide reduction to isopropanol using novel carbonized copper metal organic framework derived electrodes. J. CO2 Util. 2020, 39, 101159. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Yang, Y.-Y.; Zheng, Y.-Q.; Zhu, H.-L. Ag-doped Co3O4 catalyst derived from heterometallic MOF for syngas production by electrocatalytic reduction of CO2 in water. J. Solid State Chem. 2018, 263, 44–51. [Google Scholar] [CrossRef]
- Cao, S.-M.; Chen, H.-B.; Dong, B.-X.; Zheng, Q.-H.; Ding, Y.-X.; Liu, M.-J.; Qian, S.-L.; Teng, Y.-L.; Li, Z.-W.; Liu, W.-L. Nitrogen-rich metal-organic framework mediated Cu–N–C composite catalysts for the electrochemical reduction of CO2. J. Energy Chem. 2021, 54, 555–563. [Google Scholar] [CrossRef]
- Sun, X.; Wang, R.; Ould-Chikh, S.; Osadchii, D.; Li, G.; Aguilar, A.; Hazemann, J.-l.; Kapteijn, F.; Gascon, J. Structure-activity relationships in metal organic framework derived mesoporous nitrogen-doped carbon containing atomically dispersed iron sites for CO2 electrochemical reduction. J. Catal. 2019, 378, 320–330. [Google Scholar] [CrossRef]
- Al-Rowaili, F.N.; Jamal, A.; Ba Shammakh, M.S.; Rana, A. A Review on Recent Advances for Electrochemical Reduction of Carbon Dioxide to Methanol Using Metal–Organic Framework (MOF) and Non-MOF Catalysts: Challenges and Future Prospects. Acs Sustain. Chem. Eng. 2018, 6, 15895–15914. [Google Scholar] [CrossRef]
- Dong, B.-X.; Qian, S.-L.; Bu, F.-Y.; Wu, Y.-C.; Feng, L.-G.; Teng, Y.-L.; Liu, W.-L.; Li, Z.-W. Electrochemical Reduction of CO2 to CO by a Heterogeneous Catalyst of Fe–Porphyrin-Based Metal–Organic Framework. ACS Appl. Energy Mater. 2018, 1, 4662–4669. [Google Scholar] [CrossRef]
- Hod, I.; Sampson, M.D.; Deria, P.; Kubiak, C.P.; Farha, O.K.; Hupp, J.T. Fe-Porphyrin-Based Metal–Organic Framework Films as High-Surface Concentration, Heterogeneous Catalysts for Electrochemical Reduction of CO2. ACS Catal. 2015, 5, 6302–6309. [Google Scholar] [CrossRef]
- Wang, R.; Sun, X.; Ould-Chikh, S.; Osadchii, D.; Bai, F.; Kapteijn, F.; Gascon, J. Metal-Organic-Framework-Mediated Nitrogen-Doped Carbon for CO2 Electrochemical Reduction. ACS Appl. Mater. Interfaces 2018, 10, 14751–14758. [Google Scholar] [CrossRef]
- Witman, M.; Ling, S.; Gladysiak, A.; Stylianou, K.C.; Smit, B.; Slater, B.; Haranczyk, M. Rational Design of a Low-Cost, High-Performance Metal–Organic Framework for Hydrogen Storage and Carbon Capture. J. Phys. Chem. C 2017, 121, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, D.; Mason, J.A.; James, B.D.; Houchins, C.; Long, J.R.; Veenstra, M. Techno-economic Analysis of Metal–Organic Frameworks for Hydrogen and Natural Gas Storage. Energy Fuels 2017, 31, 2024–2032. [Google Scholar] [CrossRef]








| Molecule | Kinetic Diameter (Å) |
|---|---|
| CO2 | 3.3 |
| O2 | 3.46 |
| N2 | 3.64 |
| H2O | 2.65 |
| CH4 | 3.8 |
| H2 | 2.89 |
| MOF Name | Organic Ligand | Organic Ligand Structure |
|---|---|---|
| MOF-200 | BBC: 4,4′,4″-benzene-1,3,5-triyl-tris(benzene-4,1-diyl)tribenzoate |  |
| MOF-177 | BTB: 4,4′,4″-benzene-1,3,5-triyl-tribenzoate |  |
| MOF-180 | BTE: 4,4′,4″-benzene-1,3,5-triyl-tris(ethyne-2,1-diyl)tribenzoate |  |
| MOF-205 | BTB + 2,6-naphtalenedicarboxylate (NDC) |  |
| MOF-210 | BTE + biphenyl-4,40-dicarboxilate (BPDC) |  |
| MOFs | Liquid Amines | Amine Grafted MOFs | Zeolites | Ionic Liquids | Hybrid Ultraporous Materials (HUMs) | Soda Lime | Amine Grafted Inorganics | |
|---|---|---|---|---|---|---|---|---|
| Selectivity | Low | High | High | Low | High | Very high | High | High |
| Stability | Low | Low | Medium | High | High | Medium | High | High |
| Humidity effect | High | Low | Medium | High | Low | Medium | Low | Low |
| Material cost | Medium/high | Low | High | Low | Low | Low | Low | Medium |
| Process cost | Medium | Low | High | Low | Medium | Low | Low | Medium |
| Recycling cost | High | High | Medium | High | Medium/high | Low | Very high | Medium |
| Working capacity | High | Medium | Medium | Medium | Low | Medium | Medium | Medium |
| Kinetics | Medium | Fast | Medium | Medium | Fast | Fast | Fast | Medium |
| Upside potential | High | Low | Medium | Low | Medium | High | Low | Medium |
| Abbreviation | Name |
|---|---|
| MOF | Metal-organic frameworks |
| HUMs | Hybrid ultra-microporous materials |
| CCS | Carbon capture and storage |
| HKUST | Hong Kong university of science and technology |
| ZIF | Zeolitic imidazolate framework |
| MIL | Materials of institut lavoisier |
| TEPA | Tetraethylenepentamine |
| PIM | Polymer of intrinsic microporosity |
| TFC | Thin film composite |
| PDMS | Polydimethylsiloxane |
| PEBA | Polyether-block-amide |
| KAUST | King Abdullah university of science and technology |
| MMM | Mixed-matrix membranes |
| NPs | Nanoparticles |
| CNFs | Chitosan nanofibers |
| GO | Graphene oxide |
| PSF | Polysulfone |
| MUF | Massey university framework |
| PDA | Polydopamine |
| DABCO | Diazabicyclo octane |
| HOMO | Highest occupied molecular orbital |
| LUMO | Lowest unoccupied molecular orbital |
| PL | Photoluminescence |
| SHE | Standard hydrogen electrode |
| PIC | Porous interconnected carbon |
| FE | Faradaic efficiency |
| ECR | Electrochemical reduction |
| TMOS | Tetramethyl orthosilicate |
| CO2 | Carbon dioxide |
| N2 | Nitrogen |
| CH4 | Methane |
| H2 | Hydrogen |
| CO | Carbon monoxide |
| CH3OH | methanol |
| HCOOH | Formic acid |
| C2H4 | Ethylene |
| HCHO | Formaldehyde |
| HCOO− | Formate |
| Oxy-Combustion Carbon Capture | Pre-Combustion Carbon Capture | Post-Combustion Carbon Capture |
|---|---|---|
| Advantages | ||
| Produce high efficiency steam cycles | Frequently used in the industrial processes | Applicable for existing and new coal-fired power plants |
| Low level of Pollutants emissions at low cost | Lower energy requirements compared to other CO2 capture methods | Extensive studies are made to improve the sorbents and the capture equipment |
| Cost effective compared to other CO2 capture methods. A low cost is required to capture more than 98% of CO2 | Syngas can be used as a fuel for turbine cycle | Future developments of pulverized coal systems will increase the plant efficiency and reduce CO2 emissions |
| Easy to retrofit into an existing power plant, and does not require an on-site chemical operation | Requires less amount of water compared to post-combustion capture | Most commonly used technology in CO2 capture methods |
| Disadvantages | ||
| High Energy penalty | Significant loss of energy compared to post-combustion capture. | Low CO2 partial pressure at ambient pressure |
| High overall cost | High equipment cost | The amine technologies used results in an almost 30% loss of the net power output and an efficiency reduction of 11% |
| Technology needs to be proved for large scale operations. | Requires extensive supporting systems | The steam extraction decreases the flow to low-pressure turbine; affecting the efficiency and reducing capability |
| High risk of CO2 leakage | Mainly applicable to new plants | High performance, circulation volume, and water requirements are needed for high capture levels |
| MOF | CO2 Uptake | T (°C) | P | Ref. |
|---|---|---|---|---|
| Zn(adc) (4,40-bpe)0.5 | 130 mmol g−1 | −78.15 | 1p/p | [37] |
| (MIL-53) | 7.5 mmol g−1 | 30.85 | 20 bar | [38] |
| Cu(fam) (4,40-bpe)0.5 | 100 mL g−1 | −78.15 | 760 torr | [39] |
| Ni2(cyclam)2(mtb) | 57 mL g−1 | −78.15 | 1 atm | [40] |
| MIL-53 M = Al, Cr | 10 mmol g−1 | 30.85 | 30 bar | [41] |
| (PCN-5) | 210 mg g−1 | −78.15 | 760 torr | [42] |
| Cu(dhbc)2 (4,40-bpy) | 70 mL g−1 | 24.85 | 0.4~8 atm | [43] |
| Cu(bdc) (4,40-bpy)0.5 | 70 mL g−1 | 24.85 | 0.1~0.2 MPa | [43] |
| (ZIF-20) | 70 mL g−1 | 0 | 760 torr | [44] |
| [Ni(bpe)2 (N(CN)2)] (N(CN)2) | 35 mL g−1 | −78.15 | 1p/p | [45] |
| Zn2(tcom) (4,40-bpy) | 5 wt% | 24.85 | 1 bar | [46] |
| Cu(pyrdc)(bpp) | Differed adsorption capacity | −78.15 | Different pressure | [47] |
| Ni3(BTC)2 | 3.0 mmol g−1 | 40 | 1 bar | [48] |
| SNU-110 | 6.0 mmol g−1 | 78 | 1 bar | [49] |
| 1D-MOF | 4.0 mmol g−1 | 78 | 1 bar | [50] |
| 2D-MOF | 2.9 mmol g−1 | 0 | 1 bar | [51] |
| A core–shell MOF | 41 mmol g−1 | 0 | 1 bar | [52] |
| NJU-Bai12 | 23.8 mmol g−1 | 0 | 20 bar | [53] |
| PCN-124 | 9.1 mmol g−1 | 0 | 1 bar | [54] |
| MOF-5/graphite oxide | 1.1 mmol g−1 | 25 | 4 bar | [55] |
| HCM-Cu3(BTC)2-3 | 2.8 mmol g−1 | 25 | 1 bar | [56] |
| Zn doped Ni-ZIF-8 | 4.3 mmol g−1 | 0 | 1 bar | [57] |
| Zn(II)-based MOFs | 9.2 mmol g−1 | 25 | 1 bar | [58] |
| MOF with PEI | 4.2 mmol g−1 | 78 | 0.15 bar | [59] |
| MIL-53 with BNHx | 4.5 mmol g−1 | 0 | 1 bar | [50] |
| Mg-MOF-74 | 8.0 mmol g−1 | 23 | 1 bar | [60] |
| UMCM-1-NH2-MA | 19.8 mmol g−1 | 25 | 18 bar | [61] |
| Reduction Potentials of CO2 | Reduction Potential vs. Normal Hydrogen Electrode (NHE) (V) |
|---|---|
| CO2 + e− → CO2− | −1.9 |
| CO2 + 2H+ + 2e− → CO + H2O | −0.53 |
| CO2 + 2H+ + 2e− → HCOOH | −0.61 |
| CO2 + 4H+ + 4e− → HCHO + H2O | −0.48 |
| CO2 + 2H+ + 2e− → HCOO− | −0.49 |
| CO2 + 6H+ + 6e− → CH3OH + H2O | −0.38 |
| 2H+ + 2e− → H2 | −0.41 |
| H2O O2 + 2H+ + 2e− | +0.41 |
| CO2 + 8H+ + 8e− → HCHO + H2O | −0.24 |
| Sample ID | Proton Donor | Products and Yield (μ mol/g h) | Light Source | Reference | |||
|---|---|---|---|---|---|---|---|
| MOF4 | TEA | CO | 10.9 | _ | _ | UV | [149] |
| Zn2GeO4/ZIF-8 | H2O | CH3OH | 0.22 | _ | _ | UV | [150] |
| NH2-MIL-125(Ti) | TEOA | HCOO− | 16.3 | _ | _ | Visible | [151] |
| Cu3(BTC)2@TiO2 | H2O | CH4 a | 2.64 | _ | _ | UV | [152] |
| Copper porphyrin MOF b | TEOA | CH3OH c | 262.6 | _ | _ | Visible | [153] |
| Pt-NH2-MIL-125(Ti) Au-NH2-MIL-125(Ti) | TEOA | HCOO | 32.4 16.3 | _ | _ | [154] | |
| NH2-UiO-66(Zr) NH2-UiO-66(Zr/Ti) | TEOA | HCOO d | 3.4 5.8 | _ | _ | Visible | [155] |
| Ui-66-CrCAT Ui-66-GaCAT | TEOA | HCOOH | 1724 959 | _ | _ | [156] | |
| Co-ZIF-9 Co-MOF-74 Mn-MOF-74 Zn-ZIF-8 | TEOA | CO | 12.6 9.9 0.3 0.2 | H2 | 2.8 1.9 0.5 0.2 | Visible | [157] |
| CPO-27-Mg/TiO2 TiO2 CPO-27-Mg | H2O | CO | 4.09 2.25 0 | CH4 | 2.35 1.37 0 | UV | [158] |
| Co-ZIF-9/TiO2 | H2O | CO | 8.8 | H2 | 2.6 | UV-Vis | [159] |
| Zn/PMOF | H2O | CH4 | 8.7 | _ | _ | UV-Vis | [160] |
| PCN-22 | TEOA | HCOO | 52.8 | _ | _ | Visible | [161] |
| 2Cu/ZIF-8N2 | Na2SO3 | CH3OH e | 35.82 | _ | _ | Visible | [162] |
| Ag@Co-ZIF-9 | TEOA | CO f | 28.4 | H2 | 22.9 | Visible | [163] |
| Ni MOLs | TEOA | CO | 12.5 | H2 | 0.28 | Visible | [164] |
| TiO2/Cu2O/Cu3(BTC)2 | H2O | CO | 210 | CH4 | 160 | Visible | [165] |
| CdS/UiO-bpy/Co | TEOA | CO | 235 | _ | _ | Visible | [165] |
| NH2-rGO (5 wt%)/Al-PMOF | TEOA | HCOO | 685.6 | _ | _ | Visible | [166] |
| Zn-MOF nanosheets/ [CO2 (OH)L](ClO4)3 | TEOA | CO | 14.45 | H2 | 2.6 | Visible | [167] |
| Reduction Potentials of CO2 | Standard Electrode Potentials vs. SHE (V) |
|---|---|
| CO2 + 2H+ + 2e− → CO + H2O | −0.106 |
| 2CO2 + 2H+ + 2e− → H2C2O4 | −0.500 |
| CO2 + 2H+ + 2e− → HCOOH + H2O | −0.250 |
| CO2 + 4H+ + 4e− → CH2O + 2H2O | −0.070 |
| CO2 + 4H+ + 4e− → C + 2H2O | 0.210 |
| CO2 + 8H+ + 8e− → CH4 + 2H2O | 0.169 |
| CO2 + 6H+ + 6e− → CH3OH + H2O | 0.016 |
| CO2 + 14H+ + 14e− → C2H6 + 4H2O | 0.084 |
| CO2 + 12H+ + 12e− → C2H4 + 4H2O | 0.064 |
| Sample ID | Product | FE (%) | Potential | Reference |
|---|---|---|---|---|
| Zn-BTC | CH4 | 80.1 ± 6.6 | −2.2 V vs. Ag/AgCl | [177] |
| M-PMOF | CO | 98.7 | −0.8 V vs. RHE 1 | [178] |
| Re-SURMOF | CO | 93 ± 5 | −1.6 V vs. NHE | [179] |
| ZIF-8 | CO | 65.5 | −1.8 V vs. SCE | [180] |
| ZIF-CNT-FA-p | CO | 100 | −0.86 V vs. RHE | [181] |
| Al2(OH)2TCPP-Co | CO | 76 | −0.7 V vs. RHE | [182] |
| CR-MOF | HCOOH | 98 | −1.2 V vs. SHE | [183] |
| Ru(III)-doped HKUST1 | CH3OH, C2H5OH | 47.2 | 20 mA cm−2 | [184] |
| Ag2O/layer ZIF | CO | 80.5 | −1.2 V vs. RHE | [184] |
| C-AFC@ZIF-8 | CO | 93 | −0.6 V vs. RHE | [185] |
| ZIF-8 derived Fe-N-C | CO | 91 | −0.6 V vs. RHE | [186] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhenawy, S.E.M.; Khraisheh, M.; AlMomani, F.; Walker, G. Metal-Organic Frameworks as a Platform for CO2 Capture and Chemical Processes: Adsorption, Membrane Separation, Catalytic-Conversion, and Electrochemical Reduction of CO2. Catalysts 2020, 10, 1293. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10111293
Elhenawy SEM, Khraisheh M, AlMomani F, Walker G. Metal-Organic Frameworks as a Platform for CO2 Capture and Chemical Processes: Adsorption, Membrane Separation, Catalytic-Conversion, and Electrochemical Reduction of CO2. Catalysts. 2020; 10(11):1293. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10111293
Chicago/Turabian StyleElhenawy, Salma Ehab Mohamed, Majeda Khraisheh, Fares AlMomani, and Gavin Walker. 2020. "Metal-Organic Frameworks as a Platform for CO2 Capture and Chemical Processes: Adsorption, Membrane Separation, Catalytic-Conversion, and Electrochemical Reduction of CO2" Catalysts 10, no. 11: 1293. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10111293