Efficient Photocatalytic CO2 Reduction with MIL-100(Fe)-CsPbBr3 Composites
Abstract
:1. Introduction
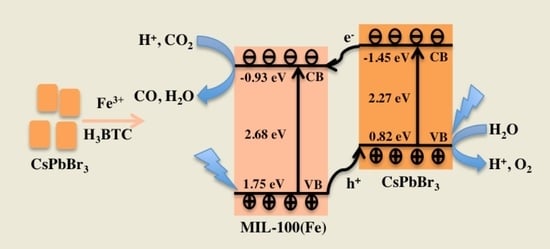
2. Results
3. Materials and Methods
3.1. Catalyst Synthesis
3.1.1. Synthesis of CsPbBr3
3.1.2. Synthesis of Pure MIL-100(Fe)
3.1.3. Synthesis of the CsPbBr3/MIL-100(Fe) Composites
3.2. Characterization
3.3. Photocatalytic CO2 Reduction Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sun, Z.X.; Wang, H.Q.; Wu, Z.B.A.; Wang, L.Z. g-C3N4 based composite photocatalysts for photocatalytic CO2 reduction. Catal. Today 2018, 300, 160–172. [Google Scholar] [CrossRef]
- Indrakanti, V.P.; Kubicki, J.D.; Schobert, H.H. Photoinduced activation of CO2 on Ti-based heterogeneous catalysts: Current state, chemical physics-based insights and outlook. Energy Environ. Sci. 2009, 2, 745–758. [Google Scholar] [CrossRef]
- Tran, P.D.; Wong, L.H.; Barber, J.; Loo, J.S.C. Recent advances in hybrid photocatalysts for solar fuel production. Energy Environ. Sci. 2012, 5, 5902–5918. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.T.; Huang, Y.; Peng, Y.W.; Huang, Z.Q.; Ma, Q.L.; Zhang, H. Two-Dimensional metal-organic framework nanosheets: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 6267–6295. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.G.; Xu, Y.; Yin, S.M.; Xu, R. Rational design of catalytic centers in crystalline frameworks. Adv. Mater. 2018, 30, 1707582–1707610. [Google Scholar] [CrossRef]
- Alvaro, M.; Carbonell, E.; Ferrer, B.; Xamena, F.X.L.I.; Garcia, H. Semiconductor behavior of a metal-organic framework (MOF). Chem. Eur. J. 2007, 13, 5106–5112. [Google Scholar] [CrossRef]
- Fu, Y.H.; Sun, D.R.; Chen, Y.J.; Huang, R.K.; Ding, Z.X.; Fu, X.Z.; Li, Z.H. An amine-functionalized titanium metal-organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew. Chem. Int. Ed. 2012, 51, 3364–3367. [Google Scholar] [CrossRef]
- Liang, Z.B.; Qu, C.; Xia, D.G.; Zou, R.Q.; Xu, Q. Atomically dispersed metal sites in MOF-based materials for electrocatalytic and photocatalytic energy conversion. Angew. Chem. Int. Ed. 2018, 57, 9604–9633. [Google Scholar] [CrossRef]
- Qiu, J.H.; Zhang, X.G.; Feng, Y.; Zhang, X.F.; Wang, H.T.; Yao, J.F. Modified metal-organic frameworks as photocatalysts. Appl. Catal. B 2018, 231, 317–342. [Google Scholar] [CrossRef]
- Yang, Z.W.; Xu, X.Q.; Liang, X.X.; Lei, C.; Wei, Y.L.; He, P.Q.; Lv, B.L.; Ma, H.C.; Lei, Z.Q. MIL-53(Fe)-graphene nanocomposites: Efficient visible-light photocatalysts for the selective oxidation of alcohols. Appl. Catal. B 2016, 198, 112–123. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Liang, X.; Shi, H.; Wang, C.; Fan, J.; Hu, X.; Liu, E. Enhanced photocatalytic activity of Ag-CsPbBr3/CN composite for broad spectrum photocatalytic degradation of cephalosporin antibiotics 7-ACA. Appl. Catal. B 2019, 247, 57–69. [Google Scholar] [CrossRef]
- Huang, H.W.; Yuan, H.F.; Janssen, K.P.F.; Solis-Fernandez, G.; Wang, Y.; Tan, C.Y.X.; Jonckheere, D.; Debroye, E.; Long, J.L.; Hendrix, J.; et al. Efficient and selective photocatalytic oxidation of benzylic alcohols with hybrid organic-inorganic perovskite materials. ACS Energy Lett. 2018, 3, 755–759. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.W.; Pradhan, B.; Hofkens, J.; Roeffaers, M.B.J.; Steele, J.A. Solar-Driven metal halide perovskite photocatalysis: Design, stability, and performance. ACS Energy Lett. 2020, 5, 1107–1123. [Google Scholar] [CrossRef]
- Kang, B.; Biswas, K. Exploring polaronic, excitonic structures and luminescence in Cs4PbBr6/CsPbBr3. J. Phys. Chem. Lett. 2018, 9, 830–836. [Google Scholar] [CrossRef]
- Dana, J.; Maity, P.; Jana, B.; Maiti, S.; Ghosh, H.N. Concurrent ultrafast electron- and hole-transfer dynamics in CsPbBr3 perovskite and quantum Dots. ACS Omega 2018, 3, 2706–2714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, R.L.; Jin, H.D.; Roeffaers, M.B.J.; Hofkens, J. Incorporation of cesium lead halide perovskites into g-C3N4 for photocatalytic CO2 Reduction. ACS Omega 2020, 5, 24495–24503. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.F.; Yang, M.Z.; Chen, B.X.; Wang, X.D.; Chen, H.Y.; Kuang, D.B.; Su, C.Y. A CsPbBr3 perovskite quantum dot/graphene oxide composite for photocatalytic CO2 reduction. J. Am. Chem. Soc. 2017, 139, 5660–5663. [Google Scholar] [CrossRef]
- Kong, Z.C.; Liao, J.F.; Dong, Y.J.; Xu, Y.F.; Chen, H.Y.; Kuang, D.B.; Su, C.Y. Core@shell CsPbBr3@zeolitic imidazolate framework nanocomposite for efficient photocatalytic CO2 reduction. ACS Energy Lett. 2018, 2656–2662. [Google Scholar] [CrossRef]
- Mollick, S.; Mandal, T.N.; Jana, A.; Fajal, S.; Desai, A.V.; Ghosh, S.K. Ultrastable luminescent hybrid bromide perovskite@MOF nanocomposites for the degradation of organic pollutants in water. ACS Appl. Nano Mater. 2019, 2, 1333–1340. [Google Scholar] [CrossRef]
- Wan, S.P.; Ou, M.; Zhong, Q.; Wang, X.M. Perovskite-type CsPbBr3 quantum dots/UiO-66(NH2) nanojunction as efficient visible-light-driven photocatalyst for CO2 reduction. Chem. Eng. J. 2019, 358, 1287–1295. [Google Scholar] [CrossRef]
- Wang, D.K.; Wang, M.T.; Li, Z.H. Fe-Based metal-organic frameworks for highly selective photocatalytic benzene hydroxylation to phenol. ACS Catal. 2015, 5, 6852–6857. [Google Scholar] [CrossRef]
- Liang, R.W.; Luo, S.G.; Jing, F.F.; Shen, L.J.; Qin, N.; Wu, L. A simple strategy for fabrication of Pd@MIL-100(Fe) nanocomposite as a visible-light-driven photocatalyst for the treatment of pharmaceuticals and personal care products (PPCPs). Appl. Catal. B 2015, 176, 240–248. [Google Scholar] [CrossRef]
- Hong, J.D.; Chen, C.P.; Bedoya, F.E.; Kelsall, G.H.; O’Hare, D.; Petit, C. Carbon nitride nanosheet/metal-organic framework nanocomposites with synergistic photocatalytic activities. Catal. Sci. Technol. 2016, 6, 5042–5051. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.J.; Jiao, Z.B. Modified Bi2WO6 with metal-organic frameworks for enhanced photocatalytic activity under visible light. J. Colloid Interface Sci. 2017, 488, 234–239. [Google Scholar] [CrossRef]
- Zhang, C.F.; Qiu, L.G.; Ke, F.; Zhu, Y.J.; Yuan, Y.P.; Xu, G.S.; Jiang, X. A novel magnetic recyclable photocatalyst based on a core-shell metal-organic framework Fe3O4@MIL-100(Fe) for the decolorization of methylene blue dye. J. Mater. Chem. A 2013, 1, 14329–14334. [Google Scholar] [CrossRef]
- Parvinizadeh, F.; Daneshfar, A. Fabrication of a magnetic metal-organic framework molecularly imprinted polymer for extraction of anti-malaria agent hydroxychloroquine. New J. Chem. 2019, 43, 8508–8516. [Google Scholar] [CrossRef]
- Chaturvedi, G.; Kaur, A.; Umar, A.; Khan, M.A.; Algarni, H.; Kansal, S.K. Removal of fluoroquinolone drug, levofloxacin, from aqueous phase over iron based MOFs, MIL-100(Fe). J. Solid State Chem. 2020, 281, 121029–121038. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J.; Oveisi, M.; Asli, M.A.; Vossoughi, M. Metal-Organic framework (MIL-100 (Fe)): Synthesis, detailed photocatalytic dye degradation ability in colored textile wastewater and recycling. Mater. Res. Bull. 2018, 100, 357–366. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.B.; Song, H.Y.; Chen, C.X.; Han, F.Q.; Wen, C.C. Protonated graphitic carbon nitride coated metal-organic frameworks with enhanced visible-light photocatalytic activity for contaminants degradation. Appl. Surf. Sci. 2018, 441, 85–98. [Google Scholar] [CrossRef]
- Li, Z.J.; Hofman, E.; Li, J.; Davis, A.H.; Tung, C.H.; Wu, L.Z.; Zheng, W.W. Photoelectrochemically active and environmentally stable CsPbBr3/TiO2 core/shell nanocrystals. Adv. Funct. Mater. 2018, 28, 1704288–1704294. [Google Scholar] [CrossRef]
- Pal, P.; Saha, S.; Banik, A.; Sarkar, A.; Biswas, K. All-solid-state mechanochemical synthesis and post-synthetic transformation of inorganic perovskite-type halides. Chem. Eur. J. 2018, 24, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Nguyen, V.; Jiang, Z.; Wang, D.W.; Zhu, Z.; Wang, W.N. Highly-Oriented one-dimensional MOF-semiconductor nanoarrays for efficient photodegradation of antibiotics. Catal. Sci. Technol. 2018, 8, 2117–2123. [Google Scholar] [CrossRef]
- Araya, T.; Chen, C.C.; Jia, M.K.; Johnson, D.; Li, R.; Huang, Y.P. Selective degradation of organic dyes by a resin modified Fe-based metal-organic framework under visible light irradiation. Opt. Mater. 2017, 64, 512–523. [Google Scholar] [CrossRef]
- Tang, X.S.; Hu, Z.P.; Yuan, W.; Hu, W.; Shao, H.B.; Han, D.J.; Zheng, J.F.; Hao, J.Y.; Zang, Z.G.; Du, J.; et al. Perovskite CsPb2Br5 microplate laser with enhanced stability and tunable properties. Adv. Opt. Mater. 2017, 5, 1600788–1600795. [Google Scholar] [CrossRef]
- Tang, X.S.; Han, S.; Zu, Z.Q.; Hu, W.; Zhou, D.; Du, J.; Hu, Z.P.; Li, S.Q.; Zang, Z.G. All-Inorganic perovskite CsPb2Br5 microsheets for photodetector application. Front. Phys. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Song, P.J.; Qiao, B.; Song, D.D.; Liang, Z.Q.; Gao, D.; Cao, J.Y.; Shen, Z.H.; Xu, Z.; Zhao, S.L. Colour- and structure-stable CsPbBr3-CsPb2Br5 compounded quantum dots with tuneable blue and green light emission. J. Alloys Compd. 2018, 767, 98–105. [Google Scholar] [CrossRef]
- Gargiulo, V.; Alfe, M.; Raganati, F.; Lisi, L.; Chirone, R.; Ammendola, P. BTC-Based metal-organic frameworks: Correlation between relevant structural features and CO2 adsorption performances. Fuel 2018, 222, 319–326. [Google Scholar] [CrossRef]
- Pu, Y.C.; Fan, H.C.; Liu, T.W.; Chen, J.W. Methylamine lead bromide perovskite/protonated graphitic carbon nitride nanocomposites: Interfacial charge carrier dynamics and photocatalysis. J. Mater. Chem. A 2017, 5, 25438–25449. [Google Scholar] [CrossRef]
- Tan, F.C.; Liu, M.; Li, K.Y.; Wang, Y.R.; Wang, J.H.; Guo, X.W.; Zhang, G.L.; Song, C.S. Facile synthesis of size-controlled MIL-100(Fe) with excellent adsorption capacity for methylene blue. Chem. Eng. J. 2015, 281, 360–367. [Google Scholar] [CrossRef]
- Zhan, X.S.; Jin, Z.W.; Zhan, J.R.; Bai, D.L.; Bian, H.; Wang, K.; Sun, J.; Wang, Q.; Liu, S.F. All-Ambient processed binary CsPbBr3-CsPb2Br5 perovskites with synergistic enhancement for high-efficiency Cs-Pb-Br-based solar cells. ACS Appl. Mater. Interfaces 2018, 10, 7145–7154. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.K.; Song, Y.J.; Cai, J.Y.; Wu, L.; Li, Z.H. Effective photo-reduction to deposit Pt nanoparticles on MIL-100(Fe) for visible-light-induced hydrogen evolution. New J. Chem. 2016, 40, 9170–9175. [Google Scholar] [CrossRef]











| Sample | p-30Fe | p-60Fe | p-90Fe | p-120Fe | p-180Fe |
|---|---|---|---|---|---|
| MIL-100(Fe) content/wt% | 9 | 14 | 18 | 27 | 53 |
| Sample | MIL-100(Fe) | CsPbBr3 | p-30Fe | p-60Fe | p-90Fe | p-120Fe | p-180Fe |
|---|---|---|---|---|---|---|---|
| Eg/eV | 2.68 | 2.27 | 2.29 | 2.29 | 2.30 | 2.55 | 2.69 |
| Sample | p-60Fe | p-90Fe | p-120Fe | p-180Fe |
|---|---|---|---|---|
| SBET/m2 g−1 | 130 | 201 | 277 | 390 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, R.; Debroye, E.; Hofkens, J.; Roeffaers, M.B.J. Efficient Photocatalytic CO2 Reduction with MIL-100(Fe)-CsPbBr3 Composites. Catalysts 2020, 10, 1352. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10111352
Cheng R, Debroye E, Hofkens J, Roeffaers MBJ. Efficient Photocatalytic CO2 Reduction with MIL-100(Fe)-CsPbBr3 Composites. Catalysts. 2020; 10(11):1352. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10111352
Chicago/Turabian StyleCheng, Ruolin, Elke Debroye, Johan Hofkens, and Maarten B. J. Roeffaers. 2020. "Efficient Photocatalytic CO2 Reduction with MIL-100(Fe)-CsPbBr3 Composites" Catalysts 10, no. 11: 1352. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10111352