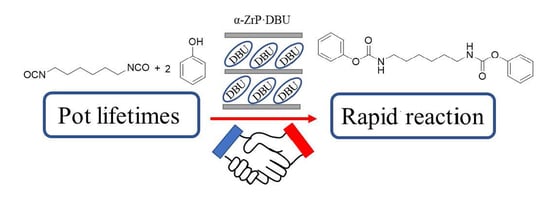
DBU-Intercalated α-Zirconium Phosphates as Latent Thermal Catalysts in the Reaction of Hexamethylene Diisocyanate and Phenol
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Measurements
3.3. Preparation of DBU-Intercalated α-ZrP (α-ZrP·0.10DBU)
3.4. Typical Model Reaction Procedure of HDI and Phenol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Compound Name |
| α-ZrP | α-zirconium phosphate |
| DBU | 1,8-diazabicyclo[5,4,0]undec-7-ene |
| HDI | hexamethylene diisocyanate |
| SA-102 | DBU salt of 2-ethylhexanoate |
References
- Alsarraf, J.; Ammar, Y.A.; Robert, F.; Cloutet, E.; Cramail, H.; Landais, Y. Cyclic guanidines as efficient organocatalysts for the synthesis of polyurethanes. Macromolecules 2012, 45, 2249–2256. [Google Scholar] [CrossRef]
- Sardon, H.; Pascual, A.; Mecerreyes, D.; Taton, D.; Cramail, H.; Hedrick, J.L. Synthesis of polyurethanes using organocatalysis: A perspective. Macromolecules 2015, 48, 3153–3165. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.P.; Boutevin, B.; Ganachaud, F. On the versatility of urethane/urea bonds: Reversibility, blocked isocyanate, and non-isocyanate polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef] [PubMed]
- Kalaimani, S.; Nasar, A.S. Catalysis of deblocking and cure reactions of easily cleavable phenol blocked polyisocyanates with poly(polytetrahydrofuran carbonate) diol. Eur. Polym. J. 2017, 91, 221–231. [Google Scholar] [CrossRef]
- Fedoseev, M.S.; Noskova, O.A.; Derzhavinskaya, L.F. Synthesis and properties of blocked Di- and polyisocyanates. Polym. Sci. Ser. D 2016, 9, 199–204. [Google Scholar] [CrossRef]
- Nasar, A.S.; Kalaimani, S. Synthesis and studies on forward and reverse reactions of phenol-blocked polyisocyanates: An insight into blocked isocyanates. RSC Adv. 2016, 6, 76802–76812. [Google Scholar] [CrossRef]
- Nakagawa, K.; Nishimura, M. Latent Crosslinking Catalysts, Their Polyurethane Compositions, and Their Two-Component, Fast-Curing, and Heat-Curable Adhesives with Long Pot Life. 2004-222703, 2006037028, 20040730. 2006. Available online: https://www.j-platpat.inpit.go.jp/p0200 (accessed on 10 May 2021).
- Zivic, N.; Sadaba, N.; Almandoz, N.; Ruipérez, F.; Mecerreyes, D.; Sardon, H. Thioxanthone-Based photobase generators for the synthesis of polyurethanes via the photopolymerization of polyols and polyisocyanates. Macromolecules 2020, 53, 2069–2076. [Google Scholar] [CrossRef]
- Alsarraf, J.; Robert, F.; Cramail, H.; Landais, Y. Latent catalysts based on guanidine templates for polyurethane synthesis. Polym. Chem. 2013, 4, 904–907. [Google Scholar] [CrossRef]
- Shimomura, O.; Sueto, R.; Kusu, H.; Ueoka, H.; Ohtaka, A.; Nomura, R. Effect of particle size of DABCO- and DBU-intercalated α-zirconium phosphate as latent thermal catalysts in the reaction of glycidyl phenyl ether (GPE) and hexahydro-4-methylphthalic anhydride (MHHPA). J. Adhes. Soc. Jpn. 2018, 54, 258–263. [Google Scholar] [CrossRef]
- Shimomura, O.; Nishisako, T.; Yamaguchi, S.; Ichihara, J.; Kirino, M.; Ohtaka, A.; Nomura, R. DABCO- and DBU-intercalated α-zirconium phosphate as latent thermal catalysts in the copolymerization of glycidyl phenyl ether (GPE) and hexahydro-4-methylphthalic anhydride (MHHPA). J. Mol. Catal. A Chem. 2016, 411, 230–238. [Google Scholar] [CrossRef]
- Shimomura, O.; Maeno, K.; Ohtaka, A.; Yamaguchi, S.; Ichihara, J.; Sakamoto, K.; Nomura, R. Alkylamines-intercalated α-zirconium phosphate as latent thermal anionic initiators. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1854–1861. [Google Scholar] [CrossRef]





| Catalyst | C (%) | H (%) | N (%) | d (Å) | Composition |
|---|---|---|---|---|---|
| a-ZrP·0.58DBU | 14.99 | 3.84 | 3.96 | 14.0 | Zr(HPO4)2·0.58DBU·2.2H2O |
| a-ZrP·0.44DBU | 11.78 | 3.30 | 3.24 | 13.4, 12.3 | Zr(HPO4)2·0.44DBU·1.7H2O |
| a-ZrP·0.22DBU | 6.38 | 2.63 | 1.75 | 12.1 | Zr(HPO4)2·0.22DBU·1.8H2O |
| a-ZrP·0.10DBU | 3.12 | 2.10 | 0.84 | 12.1 | Zr(HPO4)2·0.10DBU·1.6H2O |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimomura, O.; Kusu, H.; Ohtaka, A.; Nomura, R. DBU-Intercalated α-Zirconium Phosphates as Latent Thermal Catalysts in the Reaction of Hexamethylene Diisocyanate and Phenol. Catalysts 2021, 11, 614. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050614
Shimomura O, Kusu H, Ohtaka A, Nomura R. DBU-Intercalated α-Zirconium Phosphates as Latent Thermal Catalysts in the Reaction of Hexamethylene Diisocyanate and Phenol. Catalysts. 2021; 11(5):614. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050614
Chicago/Turabian StyleShimomura, Osamu, Hideki Kusu, Atsushi Ohtaka, and Ryôki Nomura. 2021. "DBU-Intercalated α-Zirconium Phosphates as Latent Thermal Catalysts in the Reaction of Hexamethylene Diisocyanate and Phenol" Catalysts 11, no. 5: 614. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050614