Enhanced Performance of Pt Nanoparticles on Ni-N Co-Doped Graphitized Carbon for Oxygen Reduction Reaction in Polymer Electrolyte Membrane Fuel Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology and Physical Characterization of Ni@NGC
2.2. Electrochemical Characterization of Ni@NGC
2.3. Physical Characterization of Pt/Ni@NGC
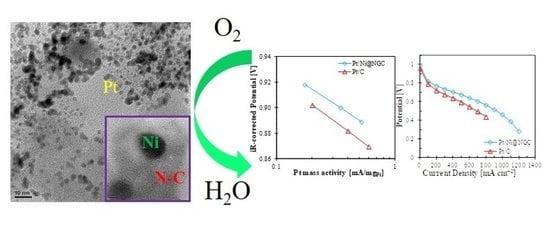
2.4. Electrochemical Characterization and Performance
3. Materials and Methods
3.1. Preparation of Ni@NGC and Pt/Ni@NGC
3.2. Physical Characterization
3.3. Electrochemical Characterization
3.4. MEA Fabrication and Single Cell Operation
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, B.; Bard, A.J. Scanning Electrochemical Microscopy. 45. Study of the Kinetics of Oxygen Reduction on Platinum with Potential Programming of the Tip. J. Phys. Chem. B 2002, 106, 12801–12806. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J. Electrocatalytic oxygen reduction reaction. In PEM Fuel Cell Electrocatalyst: Fundamentals and Applications; Zhang, J., Ed.; Springer: London, UK, 2008; pp. 89–134. [Google Scholar]
- Shao, Y.; Sui, J.; Yin, G.; Gao, Y. Nitrogen-doped carbon nanostructures and their composites as catalytic materials for proton exchange membrane fuel cell. Appl. Catal. B Environ. 2008, 79, 89–99. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Liu, H.; Li, R.; Sun, X.; Ye, S.; Knights, S. Enhanced stability of Pt electrocatalysts by nitrogen doping in CNTs for PEM fuel cells. Electrochem. Commun. 2009, 11, 2071–2076. [Google Scholar] [CrossRef]
- Lepró, X.; Terrés, E.; Vega-Cantú, Y.; Rodríguez-Macías, F.J.; Muramatsu, H.; Kim, Y.A.; Hayahsi, T.; Endo, M.; Torres, R.M.; Terrones, M. Efficient anchorage of Pt clusters on N-doped carbon nanotubes and their catalytic activity. Chem. Phys. Lett. 2008, 463, 124–129. [Google Scholar] [CrossRef]
- Wang, B. Recent development of non-platinum catalysts for oxygen reduction reaction. J. Power Sources 2005, 152, 1–15. [Google Scholar] [CrossRef]
- Nallathambi, V.; Lee, J.-W.; Kumaraguru, S.P.; Wu, G.; Popov, B.N. Development of high performance carbon composite catalyst for oxygen reduction reaction in PEM Proton Exchange Membrane fuel cells. J. Power Sources 2008, 183, 34–42. [Google Scholar] [CrossRef]
- Deshmukh, A.A.; Ul Islam, R.; Witcomb, M.J.; van Otterlo, W.A.L.; Coville, N.J. Catalytic Activity of Metal Nanoparticles Supported on Nitrogen-Doped Carbon Spheres. ChemCatChem 2010, 2, 51–54. [Google Scholar] [CrossRef]
- Higgins, D.C.; Meza, D.; Chen, Z. Nitrogen-Doped Carbon Nanotubes as Platinum Catalyst Supports for Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. J. Phys. Chem. C 2010, 114, 21982–21988. [Google Scholar] [CrossRef]
- Wang, X.; Yang, S.; Yu, Y.; Dou, M.; Zhang, Z.; Wang, F. Low-loading Pt nanoparticles embedded on Ni, N-doped carbon as superior electrocatalysts for oxygen reduction. Catal. Sci. Technol. 2020, 10, 65–69. [Google Scholar] [CrossRef]
- Jung, W.S.; Popov, B.N. Hybrid cathode catalyst with synergistic effect between carbon composite catalyst and Pt for ultra-low Pt loading in PEMFCs. Catal. Today 2017, 295, 65–74. [Google Scholar] [CrossRef]
- Karuppanan, K.K.; Raghu, A.V.; Panthalingal, M.K.; Ramanathan, S.; Kumaresan, T.; Pullithadathil, B. Triple phase boundary augmentation in hierarchical, Pt grafted N-doped mesoporous carbon nanofibers for high performance and durable PEM fuel cells. J. Mater. Chem. A 2018, 6, 12768–12781. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kwak, D.-H.; Ma, K.-B.; Han, S.-B.; Chai, G.S.; Kim, S.-K.; Peck, D.-H.; Kim, C.-S.; Kucernak, A.; Park, K.-W. Enhanced oxygen reduction reaction of Pt deposited Fe/N-doped bimodal porous carbon nanostructure catalysts. J. Catal. 2018, 359, 46–54. [Google Scholar] [CrossRef]
- Yang, H.; Ko, Y.; Lee, W.; Züttel, A.; Kim, W. Nitrogen-doped carbon black supported Pt–M (M = Pd, Fe, Ni) alloy catalysts for oxygen reduction reaction in proton exchange membrane fuel cell. Mater. Today Energy 2019, 13, 374–381. [Google Scholar] [CrossRef]
- Rethinasabapathy, M.; Kang, S.-M.; Haldorai, Y.; Jonna, N.; Jankiraman, M.; Lee, G.-W.; Jang, S.-C.; Natesan, B.; Roh, C.; Huh, Y.S. Quaternary PtRuFeCo nanoparticles supported N-doped graphene as an efficient bifunctional electrocatalyst for low-temperature fuel cells. J. Ind. Eng. Chem. 2019, 69, 285–294. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.-J.; Zhao, J.-X.; Ding, Y.-H. High stability and superior catalytic reactivity of nitrogen-doped graphene supporting Pt nanoparticles as a catalyst for the oxygen reduction reaction: A density functional theory study. RSC Adv. 2015, 5, 34070–34077. [Google Scholar] [CrossRef]
- Subramanian, N.P.; Kumaraguru, S.P.; Colon-Mercado, H.; Kim, H.; Popov, B.N.; Black, T.; Chen, D.A. Studies on Co-based catalysts supported on modified carbon substrates for PEMFC cathodes. J. Power Sources 2006, 157, 56–63. [Google Scholar] [CrossRef]
- Liu, G.; Li, X.; Ganesan, P.; Popov, B.N. Development of non-precious metal oxygen-reduction catalysts for PEM fuel cells based on N-doped ordered porous carbon. Appl. Catal. B Environ. 2009, 93, 156–165. [Google Scholar] [CrossRef]
- Li, X.; Liu, G.; Popov, B.N. Activity and stability of non-precious metal catalysts for oxygen reduction in acid and alkaline electrolytes. J. Power Sources 2010, 195, 6373–6378. [Google Scholar] [CrossRef]
- Liu, G.; Li, X.; Ganesan, P.; Popov, B.N. Studies of oxygen reduction reaction active sites and stability of nitrogen-modified carbon composite catalysts for PEM fuel cells. Electrochim. Acta 2010, 55, 2853–2858. [Google Scholar] [CrossRef]
- Li, X.; Park, S.; Popov, B.N. Highly stable Pt and PtPd hybrid catalysts supported on a nitrogen-modified carbon composite for fuel cell application. J. Power Sources 2010, 195, 445–452. [Google Scholar] [CrossRef]
- Popov, B.N.; Li, X.; Liu, G.; Lee, J.-W. Power source research at USC: Development of advanced electrocatalysts for polymer electrolyte membrane fuel cells. Int. J. Hydrog. Energy 2011, 36, 1794–1802. [Google Scholar] [CrossRef]
- Subramanian, N.P.; Li, X.; Nallathambi, V.; Kumaraguru, S.P.; Colon-Mercado, H.; Wu, G.; Lee, J.-W.; Popov, B.N. Nitrogen-modified carbon-based catalysts for oxygen reduction reaction in polymer electrolyte membrane fuel cells. J. Power Sources 2009, 188, 38–44. [Google Scholar] [CrossRef]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon Materials for Chemical Capacitive Energy Storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef]
- Su, F.; Zeng, J.; Bao, X.; Yu, Y.; Lee, J.Y.; Zhao, X.S. Preparation and Characterization of Highly Ordered Graphitic Mesoporous Carbon as a Pt Catalyst Support for Direct Methanol Fuel Cells. Chem. Mater. 2005, 17, 3960–3967. [Google Scholar] [CrossRef]
- Jung, W.S. High-performance bimetallic alloy catalyst using Ni and N co-doped composite carbon for the oxygen electro-reduction. J. Colloid Interface Sci. 2018, 514, 30–39. [Google Scholar] [CrossRef]
- Jung, W.S.; Popov, B.N. Improved durability of Pt catalyst supported on N-doped mesoporous graphitized carbon for oxygen reduction reaction in polymer electrolyte membrane fuel cells. Carbon 2017, 122, 746–755. [Google Scholar] [CrossRef]
- Wong, W.Y.; Daud, W.R.W.; Mohamad, A.B.; Kadhum, A.A.H.; Majlan, E.H.; Loh, K.S. Nitrogen-containing carbon nanotubes as cathodic catalysts for proton exchange membrane fuel cells. Diam. Relat. Mater. 2012, 22, 12–22. [Google Scholar] [CrossRef]
- Matter, P.H.; Zhang, L.; Ozkan, U.S. The role of nanostructure in nitrogen-containing carbon catalysts for the oxygen reduction reaction. J. Catal. 2006, 239, 83–96. [Google Scholar] [CrossRef]
- Niwa, H.; Horiba, K.; Harada, Y.; Oshima, M.; Ikeda, T.; Terakura, K.; Ozaki, J.-I.; Miyata, S. X-ray absorption analysis of nitrogen contribution to oxygen reduction reaction in carbon alloy cathode catalysts for polymer electrolyte fuel cells. J. Power Sources 2009, 187, 93–97. [Google Scholar] [CrossRef]
- Kangasniemi, K.H.; Condit, D.A.; Jarvi, T.D. Characterization of Vulcan Electrochemically Oxidized under Simulated PEM Fuel Cell Conditions. J. Electrochem. Soc. 2004, 151, E125–E132. [Google Scholar] [CrossRef]
- Ye, J.-S.; Liu, X.; Cui, H.F.; Zhang, W.-D.; Sheu, F.-S.; Lim, T.M. Electrochemical oxidation of multi-walled carbon nanotubes and its application to electrochemical double layer capacitors. Electrochem. Commun. 2005, 7, 249–255. [Google Scholar] [CrossRef]
- Wang, J.; Yin, G.; Shao, Y.; Zhang, S.; Wang, Z.; Gao, Y. Effect of carbon black support corrosion on the durability of Pt/C catalyst. J. Power Sources 2007, 171, 331–339. [Google Scholar] [CrossRef]
- Chen, J.H.; Li, W.Z.; Wang, D.Z.; Yang, S.X.; Wen, J.G.; Ren, Z.F. Electrochemical characterization of carbon nanotubes as electrode in electrochemical double-layer capacitors. Carbon 2002, 40, 1193–1197. [Google Scholar] [CrossRef]
- Jiang, R.; Anson, F.C. The origin of inclined plateau currents in steady-state voltammograms for electrode processes involving electrocatalysis. J. Electroanal. Chem. Interf. Electrochem. 1991, 305, 171–184. [Google Scholar] [CrossRef]
- Gojković, S.L.; Zečević, S.K.; Savinell, R.F. O2 Reduction on an Ink-Type Rotating Disk Electrode Using Pt Supported on High-Area Carbons. J. Electrochem. Soc. 1998, 145, 3713–3720. [Google Scholar] [CrossRef]
- Zhang, J.; Mo, Y.; Vukmirovic, M.B.; Klie, R.; Sasaki, K.; Adzic, R.R. Platinum Monolayer Electrocatalysts for O2 Reduction: Pt Monolayer on Pd(111) and on Carbon-Supported Pd Nanoparticles. J. Phys. Chem. B 2004, 108, 10955–10964. [Google Scholar] [CrossRef]
- Wang, C.H.; Du, H.Y.; Tsai, Y.T.; Chen, C.P.; Huang, C.J.; Chen, L.C.; Chen, K.H.; Shih, H.C. High performance of low electrocatalysts loading on CNT directly grown on carbon cloth for DMFC. J. Power Sources 2007, 171, 55–62. [Google Scholar] [CrossRef]
- Sjöström, H.; Stafström, S.; Boman, M.; Sundgren, J.E. Superhard and Elastic Carbon Nitride Thin Films Having Fullerenelike Microstructure. Phys. Rev. Lett. 1995, 75, 1336–1339. [Google Scholar] [CrossRef]
- Jia, R.L.; Wang, C.Y.; Wang, S.M. Preparation of carbon supported platinum catalysts: Role of π sites on carbon support surface. J. Mater. Sci. 2006, 41, 6881–6888. [Google Scholar] [CrossRef]
- Jafri, R.I.; Rajalakshmi, N.; Ramaprabhu, S. Nitrogen doped graphene nanoplatelets as catalyst support for oxygen reduction reaction in proton exchange membrane fuel cell. J. Mater. Chem. 2010, 20, 7114–7117. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, C.; Ji, G.; Lee, J.Y. Nitrogen-doped carbon-encapsulation of Fe3O4 for increased reversibility in Li+ storage by the conversion reaction. J. Mater. Chem. 2012, 22, 7845–7850. [Google Scholar] [CrossRef]
- Fujisawa, K.; Tojo, T.; Muramatsu, H.; Elías, A.L.; Vega-Díaz, S.M.; Tristán-López, F.; Kim, J.H.; Hayashi, T.; Kim, Y.A.; Endo, M.; et al. Enhanced electrical conductivities of N-doped carbon nanotubes by controlled heat treatment. Nanoscale 2011, 3, 4359–4364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groves, M.N.; Chan, A.S.W.; Malardier-Jugroot, C.; Jugroot, M. Improving platinum catalyst binding energy to graphene through nitrogen doping. Chem. Phys. Lett. 2009, 481, 214–219. [Google Scholar] [CrossRef]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, W.S. Enhanced Performance of Pt Nanoparticles on Ni-N Co-Doped Graphitized Carbon for Oxygen Reduction Reaction in Polymer Electrolyte Membrane Fuel Cells. Catalysts 2021, 11, 909. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080909
Jung WS. Enhanced Performance of Pt Nanoparticles on Ni-N Co-Doped Graphitized Carbon for Oxygen Reduction Reaction in Polymer Electrolyte Membrane Fuel Cells. Catalysts. 2021; 11(8):909. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080909
Chicago/Turabian StyleJung, Won Suk. 2021. "Enhanced Performance of Pt Nanoparticles on Ni-N Co-Doped Graphitized Carbon for Oxygen Reduction Reaction in Polymer Electrolyte Membrane Fuel Cells" Catalysts 11, no. 8: 909. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080909