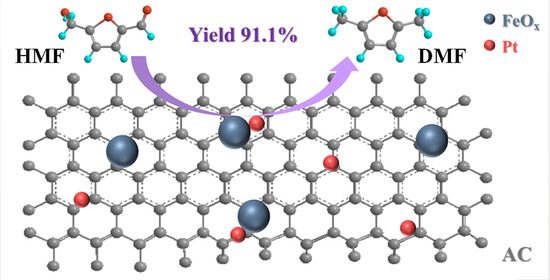
Selective 5-Hydroxymethylfurfural Hydrogenolysis to 2,5-Dimethylfuran over Bimetallic Pt-FeOx/AC Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Catalytic Performance
2.3. Catalyst Stability
3. Materials and Methods
3.1. Materials
3.2. Catalysts Preparation
3.3. Catalyst Characterization
3.4. Catalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, F.; Mao, J.; Li, S.; Yin, J.; Zhou, J.; Liu, W. Cobalt–graphene nanomaterial as an efficient catalyst for selective hydrogenation of 5-hydroxymethylfurfural into 2,5-dimethylfuran. Catal. Sci. Technol. 2019, 9, 1329–1333. [Google Scholar] [CrossRef]
- Tan, J.; Cui, J.; Zhu, Y.; Cui, X.; Shi, Y.; Yan, W.; Zhao, Y. Complete Aqueous Hydrogenation of 5-Hydroxymethylfurfural at Room Temperature over Bimetallic RuPd/Graphene Catalyst. ACS Sustain. Chem. Eng. 2019, 7, 10670–10678. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, C.; Li, D.; Liu, Q.; Tan, J.; Wang, C.; Cai, C.; Ma, L. Recent advances in catalytic conversion of biomass to 5-hydroxymethylfurfural and 2, 5-dimethylfuran. Renew. Sustain. Energy Rev. 2019, 103, 227–247. [Google Scholar] [CrossRef]
- Fulignati, S.; Antonetti, C.; Licursi, D.; Pieraccioni, M.; Wilbers, E.; Heeres, H.J.; Raspolli Galletti, A.M. Insight into the hydrogenation of pure and crude HMF to furan diols using Ru/C as catalyst. Appl. Catal. A 2019, 578, 122–133. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Gou, X.; Chen, H.; Chen, K.; Lu, X.; Ouyang, P.; Fu, J. Catalytic in-situ hydrogenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran over Cu-based catalysts with methanol as a hydrogen donor. Appl. Catal. A 2019, 570, 245–250. [Google Scholar] [CrossRef]
- Sarkar, C.; Koley, P.; Shown, I.; Lee, J.; Liao, Y.F.; An, K.; Tardio, J.; Nakka, L.; Chen, K.H.; Mondal, J. Integration of Interfacial and Alloy Effects to Modulate Catalytic Performance of Metal–Organic-Framework-Derived Cu–Pd Nanocrystals toward Hydrogenolysis of 5-Hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2019, 7, 10349–10362. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Li, S.; Chen, C.; Wu, T.; Mei, Q.; Wang, Y.; Chen, B.; Liu, H.; Han, B. Hydrogenolysis of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran under Mild Conditions without Any Additive. ACS Sustain. Chem. Eng. 2019, 7, 5711–5716. [Google Scholar] [CrossRef]
- Li, J.; Song, Z.; Hou, Y.; Li, Z.; Xu, C.; Liu, C.; Dong, W. Direct Production of 2,5-Dimethylfuran with High Yield from Fructose over a Carbon-Based Solid Acid-Coated CuCo Bimetallic Catalyst. ACS Appl. Mater. Interfaces 2019, 11, 12481–12491. [Google Scholar] [CrossRef]
- Solanki, B.S.; Rode, C.V. Selective hydrogenolysis of 5-(hydroxymethyl)furfural over Pd/C catalyst to 2,5-dimethylfuran. J. Saudi Chem. Soc. 2019, 23, 439–451. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, S.; Wang, C.; Liu, M.; Zhang, F.; Hu, X.; Chen, H.; Gou, X.; Chen, K.; Zhu, Y.; et al. CuZnCoOx multifunctional catalyst for in situ hydrogenation of 5-hydroxymethylfurfural with ethanol as hydrogen carrier. J. Catal. 2019, 373, 314–321. [Google Scholar] [CrossRef]
- Talpade, A.D.; Tiwari, M.S.; Yadav, G.D. Selective hydrogenation of bio-based 5-hydroxymethyl furfural to 2,5-dimethylfuran over magnetically separable Fe-Pd/C bimetallic nanocatalyst. Mol. Catal. 2019, 465, 1–15. [Google Scholar] [CrossRef]
- Hu, L.; Tang, X.; Xu, J.; Wu, Z.; Lin, L.; Liu, S. Selective Transformation of 5-Hydroxymethylfurfural into the Liquid Fuel 2,5-Dimethylfuran over Carbon-Supported Ruthenium. Ind. Eng. Chem. Res. 2014, 53, 3056–3064. [Google Scholar] [CrossRef]
- Priecel, P.; Endot, N.A.; Carà, P.D.; Lopez-Sanchez, J.A. Fast Catalytic Hydrogenation of 2,5-Hydroxymethylfurfural to 2,5-Dimethylfuran with Ruthenium on Carbon Nanotubes. Ind. Eng. Chem. Res. 2018, 57, 1991–2002. [Google Scholar] [CrossRef]
- Luo, J.; Arroyo-Ramírez, L.; Gorte, R.J.; Tzoulaki, D.; Vlachos, D.G. Hydrodeoxygenation of HMF over Pt/C in a continuous flow reactor. AlChE J. 2015, 61, 590–597. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Yu, X.; Du, W.; Hou, Z. Production of 2,5-dimethylfuran from 5-hydroxymethylfurfural over reduced graphene oxides supported Pt catalyst under mild conditions. Fuel 2016, 163, 74–79. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ishizaka, T.; Kawanami, H. Hydrogenation of 5-hydroxymethylfurfural in supercritical carbon dioxide–water: A tunable approach to dimethylfuran selectivity. Green Chem. 2014, 16, 1543–1551. [Google Scholar] [CrossRef]
- Gyngazova, M.S.; Negahdar, L.; Blumenthal, L.C.; Palkovits, R. Experimental and kinetic analysis of the liquid phase hydrodeoxygenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran over carbon-supported nickel catalysts. Chem. Eng. Sci. 2017, 173, 455–464. [Google Scholar] [CrossRef]
- Goyal, R.; Sarkar, B.; Bag, A.; Siddiqui, N.; Dumbre, D.; Lucas, N.; Bhargava, S.K.; Bordoloi, A. Studies of synergy between metal–support interfaces and selective hydrogenation of HMF to DMF in water. J. Catal. 2016, 340, 248–260. [Google Scholar] [CrossRef]
- Li, D.; Liu, Q.; Zhu, C.; Wang, H.; Cui, C.; Wang, C.; Ma, L. Selective hydrogenolysis of 5-hydroxymethylfurfural to 2,5-dimethylfuran over Co3O4 catalyst by controlled reduction. J. Energy Chem. 2019, 30, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Hansen, T.S.; Barta, K.; Anastas, P.T.; Ford, P.C.; Riisager, A. One-pot reduction of 5-hydroxymethylfurfural via hydrogen transfer from supercritical methanol. Green Chem. 2012, 14, 2457–2461. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Lee, J.D.; Yun, H.; Wang, C.; Monai, M.; Murray, C.B.; Fornasiero, P.; Gorte, R.J. Base metal-Pt alloys: A general route to high selectivity and stability in the production of biofuels from HMF. Appl. Catal. B 2016, 199, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Esen, M.; Akmaz, S.; Koç, S.N.; Gürkaynak, M.A. The hydrogenation of 5-hydroxymethylfurfural (HMF) to 2,5-dimethylfuran (DMF) with sol–gel Ru-Co/SiO2 catalyst. J. Sol.-Gel Sci. Technol. 2019, 91, 664–672. [Google Scholar] [CrossRef]
- Wang, G.; Hilgert, J.; Richter, F.H.; Wang, F.; Bongard, H.J.; Spliethoff, B.; Weidenthaler, C.; Schüth, F. Platinum–cobalt bimetallic nanoparticles in hollow carbon nanospheres for hydrogenolysis of 5-hydroxymethylfurfural. Nat. Mater. 2014, 13, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Ikeda, N.; Ebitani, K. Selective hydrogenation of biomass-derived 5-hydroxymethylfurfural (HMF) to 2,5-dimethylfuran (DMF) under atmospheric hydrogen pressure over carbon supported PdAu bimetallic catalyst. Catal. Today 2014, 232, 89–98. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Yuan, F.; Niu, X.; Zhu, Y. Efficient Production of the Liquid Fuel 2,5-Dimethylfuran from 5-Hydroxymethylfurfural in the Absence of Acid Additive over Bimetallic PdAu Supported on Graphitized Carbon. Energy Fuels 2017, 31, 6364–6373. [Google Scholar] [CrossRef]
- Gawade, A.B.; Tiwari, M.S.; Yadav, G.D. Biobased Green Process: Selective Hydrogenation of 5-Hydroxymethylfurfural to 2,5-Dimethyl Furan under Mild Conditions Using Pd-Cs2.5H0.5PW12O40/K-10 Clay. ACS Sustain. Chem. Eng. 2016, 4, 4113–4123. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Cai, C.; Tan, J.; Wang, T.; Ma, L. Advances in DMF and C5/C6 Alkanes Production from Lignocellulose. Prog. Chem. 2016, 28, 363–374. [Google Scholar]
- Yang, Y.; Liu, Q.; Li, D.; Tan, J.; Zhang, Q.; Wang, C.; Ma, L. Selective hydrodeoxygenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran on Ru–MoOx/C catalysts. RSC Adv. 2017, 7, 16311–16318. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhou, M.; Wang, A.; Zhang, T. Selective Hydrogenation over Supported Metal Catalysts: From Nanoparticles to Single Atoms. Chem. Rev. 2019, 120, 683–733. [Google Scholar] [CrossRef]
- Wang, X.; Liang, X.; Li, J.; Li, Q. Catalytic hydrogenolysis of biomass-derived 5-hydroxymethylfurfural to biofuel 2, 5-dimethylfuran. Appl. Catal. A 2019, 576, 85–95. [Google Scholar] [CrossRef]
- Yu, L.; He, L.; Chen, J.; Zheng, J.; Ye, L.; Lin, H.; Yuan, Y. Robust and Recyclable Nonprecious Bimetallic Nanoparticles on Carbon Nanotubes for the Hydrogenation and Hydrogenolysis of 5-Hydroxymethylfurfural. ChemCatChem 2015, 7, 1701–1707. [Google Scholar] [CrossRef]
- Shi, Y.; Yuan, Z.; Wei, Q.; Sun, K.; Xu, B. Pt–FeOx/SiO2 catalysts prepared by galvanic displacement show high selectivity for cinnamyl alcohol production in the chemoselective hydrogenation of cinnamaldehyde. Catal. Sci. Technol. 2016, 6, 7033–7037. [Google Scholar] [CrossRef]
- Wan, W.; Jiang, Z.; Chen, J.G. A Comparative Study of Hydrodeoxygenation of Furfural Over Fe/Pt(111) and Fe/Mo2C Surfaces. Top. Catal. 2018, 61, 439–445. [Google Scholar] [CrossRef]
- Zanuttini, M.S.; Gross, M.; Marchetti, G.; Querini, C. Furfural hydrodeoxygenation on iron and platinum catalysts. Appl. Catal. A 2019, 587, 117217. [Google Scholar] [CrossRef]
- Cui, W.; Xue, D.; Tan, N.; Zheng, B.; Jia, M.; Zhang, W. Pt supported on octahedral Fe3O4 microcrystals as a catalyst for removal of formaldehyde under ambient conditions. Chin. J. Catal. 2018, 39, 1534–1542. [Google Scholar]
- Guo, C.; Lu, W.; Wei, G.; Jiang, L.; Yu, Y.; Hu, Y. Formation of 1D chain-like Fe3O4@C/Pt sandwich nanocomposites and their magnetically recyclable catalytic property. Appl. Surf. Sci. 2018, 457, 1136–1141. [Google Scholar] [CrossRef]
- Liu, H.; Tian, K.; Ning, J.; Zhong, Y.; Zhang, Z.; Hu, Y. One-Step Solvothermal Formation of Pt Nanoparticles Decorated Pt2+-Doped α-Fe2O3 Nanoplates with Enhanced Photocatalytic O2 Evolution. ACS Catal. 2019, 9, 1211–1219. [Google Scholar] [CrossRef]
- Pan, H.; Li, J.; Lu, J.; Wang, G.; Xie, W.; Wu, P.; Li, X. Selective hydrogenation of cinnamaldehyde with PtFe/Al2O3@SBA-15 catalyst: Enhancement in activity and selectivity to unsaturated alcohol by Pt-FeOx and Pt-Al2O3@SBA-15 interaction. J. Catal. 2017, 354, 24–36. [Google Scholar] [CrossRef]
- Wei, M.; Han, Y.; Liu, Y.; Su, B.; Yang, H.; Lei, Z. Green preparation of Fe3O4 coral-like nanomaterials with outstanding magnetic and OER properties. J. Alloys Compd. 2020, 831, 154702. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, W.; Wan, X.; Meng, Y.; Yao, Y.; Guo, Z.; Dai, Y.; Wang, C.; Yang, Y. Oxidation of 5-hydroxymethylfurfural over a magnetic iron oxide decorated rGO supporting Pt nanocatalyst. Catal. Today 2019, 330, 92–100. [Google Scholar] [CrossRef]
- Neumann, S.; Doebler, H.H.; Keil, S.; Erdt, A.J.; Gutsche, C.; Borchert, H.; Kolny-Olesiak, J.; Parisi, J.; Bäumer, M.; Kunz, S. Effects of Particle Size on Strong Metal–Support Interactions Using Colloidal “Surfactant-Free” Pt Nanoparticles Supported on Fe3O4. ACS Catal. 2020, 10, 4136–4150. [Google Scholar] [CrossRef]
- Sarno, M.; Ponticorvo, E. Much enhanced electrocatalysis of Pt/PtO2 and low platinum loading Pt/PtO2-Fe3O4 dumbbell nanoparticles. Int. J. Hydrogen Energy 2017, 42, 23631–23638. [Google Scholar] [CrossRef]
- Tian, M.; Liu, S.; Wang, L.; Ding, H.; Zhao, D.; Wang, Y.; Cui, J.; Fu, J.; Shang, J.; Li, G.K. Complete Degradation of Gaseous Methanol over Pt/FeOx Catalysts by Normal Temperature Catalytic Ozonation. Environ. Sci. Technol. 2020, 54, 1938–1945. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, C.; Niu, T.; Huang, R.; Li, S.; Sun, J.; Wang, Y. Facile synthesis of reusable magnetic Fe/Fe3C/C composites from renewable resources for super-fast removal of organic dyes: Characterization, mechanism and kinetics. Powder Technol. 2019, 351, 314–324. [Google Scholar] [CrossRef]
- Yang, S.; Chen, L.; Mu, L.; Hao, B.; Chen, J.; Ma, P. Graphene foam with hierarchical structures for the removal of organic pollutants from water. RSC Adv. 2016, 6, 4889–4898. [Google Scholar] [CrossRef]
- Fedyna, M.; Żak, A.; Jaroszewska, K.; Mokrzycki, J.; Trawczyński, J. Composite of Pt/AlSBA-15+zeolite catalyst for the hydroisomerization of n-hexadecane: The effect of platinum precursor. Microporous Mesoporous Mater. 2020, 305, 110366. [Google Scholar] [CrossRef]
- Jystad, A.; Leblanc, H.; Caricato, M. Surface Acidity Characterization of Metal-Doped Amorphous Silicates via Py-FTIR and 15N NMR Simulations. J. Phys. Chem. C 2020, 124, 15231–15240. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Liu, H.; Liu, J.; Xu, G.; Liu, J.; Sun, H.; Fu, Y. Graphitic Carbon Nitride (g-C3N4)-derived Fe-N-C Catalysts for Selective Hydrodeoxygenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran. ChemistrySelect 2017, 2, 11062–11070. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, H.; Li, H.; Cai, B.; Lv, W.; Cai, C.; Wang, C.; Yan, L.; Liu, Q.; Ma, L. Selective Hydrodeoxygenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran over Alloyed Cu−Ni Encapsulated in Biochar Catalysts. ACS Sustain. Chem. Eng. 2019, 7, 19556–19569. [Google Scholar] [CrossRef]
- Simonsen, S.B.; Chorkendorff, I.; Dahl, S.; Skoglundh, M.; Sehested, J.; Helveg, S. Ostwald ripening in a Pt/SiO2 model catalyst studied by in situ TEM. J. Catal. 2011, 281, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Shao, M.; Hu, C.; Xu, X.; Song, Y.; Zhu, Q. Pt/TS-1 catalysts: Effect of the platinum loading method on the dehydrogenation of n-butane. Appl. Catal. A 2021, 621, 118194. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of intergrated molar extinction coefficients for infrared adsorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Ning, P.; Song, Z.; Li, H.; Zhang, Q.; Liu, X.; Zhang, J.; Tang, X.; Huang, Z. Selective catalytic reduction of NO with NH3 over CeO2–ZrO2–WO3 catalysts prepared by different methods. Appl. Surf. Sci. 2015, 332, 130–137. [Google Scholar] [CrossRef]










| Catalysts | dPt a (nm) | DPt b | Pt c (wt %) | Ptsur d (μmol/g) | Fe c (wt %) | Lewis Acidic Sites e (μmol/g) | Fe2+/Fe3+ f | Pt2+/Pt0 f |
|---|---|---|---|---|---|---|---|---|
| 0.5%Pt/AC | 4.7 ± 1.32 | 0.24 | 0.35 | 4.3 | - | 15.6 | - | 0.61 |
| 2%FeOx/AC | - | - | - | - | 1.9 | 16.5 | 0.25 | - |
| 0.1%Pt-2%FeOx/AC | 1.7 ± 0.25 | 0.67 | 0.06 | 2.1 | 1.6 | 22.5 | 0.31 | 0.98 |
| 0.3%Pt-2%FeOx/AC | 1.8 ± 0.26 | 0.63 | 0.21 | 6.8 | 1.5 | 26.2 | 0.47 | 0.91 |
| 0.5%Pt-1%FeOx/AC | 4.3 ± 1.17 | 0.26 | 0.45 | 6.0 | 1.0 | 20.9 | 0.99 | 0.73 |
| 0.5%Pt-2%FeOx/AC | 2.8 ± 0.67 | 0.40 | 0.36 | 7.4 | 1.6 | 29.9 | 0.89 | 0.82 |
| Used-1st 0.5%Pt-2%FeOx/AC | 3.3 ± 1.03 | 0.34 | 0.35 | 6.1 | 1.2 | - | 0.52 | 0.14 |
| 0.5%Pt-5.83%FeOx/AC | 4.6 ± 1.26 | 0.25 | 0.33 | 4.2 | 4.6 | 16.4 | 0.66 | 1.23 |
| Catalysts | HMF Conversion (mol %) | Yield (Selectivity) a (mol %) | Carbon Balance b (mol %) | ||||
|---|---|---|---|---|---|---|---|
DMF | DMTHF | MF | MFA | BHMF | |||
| 0.5%Pt/AC | 70.3 | 36.1 (51.3) | 3.9 (5.5) | 15.5 (22.0) | 6.2 (8.8) | - | 87.8 |
| 2%FeOx/AC | 30.7 | - | - | 2.4 (7.8) | - | 7.8 | |
| 0.1%Pt-2%FeOx/AC | 44.1 | 2.6 (5.9) | - | 13.4 (30.3) | 7.9 (17.9) | - | 54.2 |
| 0.3%Pt-2%FeOx/AC | 56.2 | 8.1 (14.4) | - | 26.4 (47.0) | 11.1 (19.7) | - | 81.1 |
| 0.5%Pt-1%FeOx/AC | 80.3 | 42.3 (52.7) | 3.1 (3.9) | 20.5 (25.5) | 5.0 (6.2) | - | 88.3 |
| 0.5%Pt-2%FeOx/AC | 86.4 | 46.5 (53.8) | - | 23.8 (27.5) | 7.6 (8.8) | - | 90.2 |
| 0.5%Pt-5.83% FeOx/AC | 65.3 | 36.9 (56.5) | - | 18.1 (27.7) | 2.7 (4.1) | - | 88.4 |
| Entry | Substrate | Conversion (mol %) | Product yield (mol %) | ||
|---|---|---|---|---|---|
| DMF | MF | MFA | |||
| 1 | BHMF | 21.7 | 9.5 | 0 | 5.3 |
| 2 | MF | 82.5 | 61.7 | 17.5 | 19.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, Y.; Li, S.; Wang, H.; Chen, L.; Li, S.; Liu, Q. Selective 5-Hydroxymethylfurfural Hydrogenolysis to 2,5-Dimethylfuran over Bimetallic Pt-FeOx/AC Catalysts. Catalysts 2021, 11, 915. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080915
Xin Y, Li S, Wang H, Chen L, Li S, Liu Q. Selective 5-Hydroxymethylfurfural Hydrogenolysis to 2,5-Dimethylfuran over Bimetallic Pt-FeOx/AC Catalysts. Catalysts. 2021; 11(8):915. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080915
Chicago/Turabian StyleXin, Yongjie, Sichan Li, Haiyong Wang, Lungang Chen, Shuang Li, and Qiying Liu. 2021. "Selective 5-Hydroxymethylfurfural Hydrogenolysis to 2,5-Dimethylfuran over Bimetallic Pt-FeOx/AC Catalysts" Catalysts 11, no. 8: 915. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080915