Graded Preparation and Industrial Applications of Large-Ball Polyolefin Catalyst Carriers
Abstract
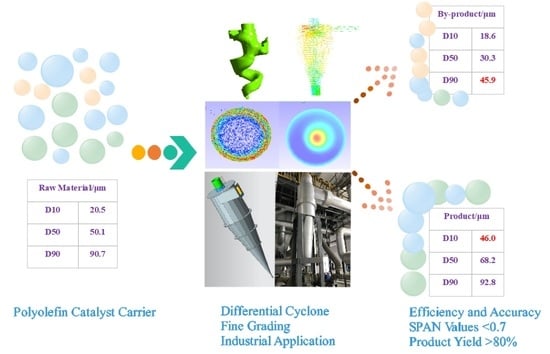
:1. Introduction
2. Experimental
2.1. Carrier Particle Classification Process
2.2. Measurement
3. Results and Discussion
3.1. Spherical Carrier Fine Grade Amplification
3.1.1. Process Flow
50-μm Hydrocyclone Separation after Washing the Kettle
Increase the Hydrocyclone Separation Transition Kettle
Increased Solid Content Preparation Large Particle Size Carrier for the 50-μm Hydrocyclone Separation
Production Feeding Conditions
3.1.2. Analysis and Discussion of Test Results
Directly Separating by the 50-μm Hydrocyclone after Washing
The Rotary Flow Separation Intermediate Kettle
Increasing Solid Content to Prepare Large Particle Size Carrier by 50-μm Hydrocyclone Separator
50-μm Hydrocyclone Separation Process vs. Screening Process
Spin-Off Carrier Preparation Catalyst
Hydrocyclone Separation Process Feasibility of Industrial Applications
3.2. Large Particle Size Carrier System (Technical Optimization of Large Particle Size System Preparation)
3.2.1. Experiment Scenario
Introduction of the Key Equipment for the Formation of Large Spherical Catalyst Carrier—Hypergravity Machine
Pilot Technical Scheme
3.2.2. Test Results Discussion and Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Larsson, P.-O.; Åkesson, J.; Carlsson, N.; Andersson, N. Model-based optimization of economical grade changes for the Borealis Borstar® polyethylene plant. Comput. Chem. Eng. 2012, 46, 153–166. [Google Scholar] [CrossRef]
- Soares, J.B.P. Mathematical modelling of the microstructure of polyolefins made by coordination polymerization: A review. Chem. Eng. Sci. 2001, 56, 4131–4153. [Google Scholar] [CrossRef]
- Covezzi, M.; Mei, G. The multizone circulating reactor technology. Chem. Eng. Sci. 2001, 56, 4059–4067. [Google Scholar] [CrossRef]
- Luo, Z.-H.; Su, P.-L.; Shi, D.-P.; Zheng, Z.-W. Steady-state and dynamic modeling of commercial bulk polypropylene process of Hypol technology. Chem. Eng. J. 2009, 149, 370–382. [Google Scholar] [CrossRef]
- Pluta, T.; Sadlej, A.J. HyPol basis sets for high-level-correlated calculations of electric dipole hyperpolarizabilities. Chem. Phys. Lett. 1998, 297, 391–401. [Google Scholar] [CrossRef]
- Urdampilleta, I.; González, A.; Iruin, J.J.; de la Cal, J.C.; Asua, J.M. Origins of product heterogeneity in the spheripol high impact polypropylene process. Ind. Eng. Chem. Res. 2006, 45, 4178–4187. [Google Scholar] [CrossRef]
- Zheng, Z.-W.; Shi, D.-P.; Su, P.-L.; Luo, Z.-H.; Li, X.-J. Steady-state and dynamic modeling of the basell multireactor olefin polymerization process. Ind. Eng. Chem. Res. 2011, 50, 322–331. [Google Scholar] [CrossRef]
- Luo, Z.-H.; Su, P.-L.; Wu, W. Industrial loop reactor for catalytic propylene polymerization: Dynamic modeling of emergency accidents. Ind. Eng. Chem. Res. 2010, 49, 11232–11243. [Google Scholar] [CrossRef]
- UNIPOL PP Process Technology Used in Coal-to-Olefins Plant in China. China Chem. Rep. 2010, 21, 11.
- Lau, C.K.; Heng, Y.S.; Hussain, M.A.; Nor, M.I.M. Fault diagnosis of the polypropylene production process (UNIPOL PP) using ANFIS. ISA Trans. 2010, 49, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Zacca, J.J.; Debling, J.A.; Ray, W.H. Reactor residence time distribution effects on the multistage polymerization of olefins—I. Basic principles and illustrative examples, polypropylene. Chem. Eng. Sci. 1996, 51, 4859–4886. [Google Scholar] [CrossRef]
- Alperowicz, N. Novolen technology likely for mangalore PP Complex. Chem. Week 2009, 171, 27. [Google Scholar]
- Amec, I.Y. Foster Wheeler, Novolen Win Big Awards in China. Chem. Week 2006, 168, 20. [Google Scholar]
- Cullivan, J.C.; Williams, R.A.; Cross, R. Understanding the hydrocyclone separator through computational fluid dynamics. Chem. Eng. Res. Des. 2003, 81, 455–466. [Google Scholar] [CrossRef]
- Wu, S.-E.; Hwang, K.-J.; Cheng, T.-W.; Hung, T.-C.; Tung, K.-L. Effectiveness of a hydrocyclone in separating particles suspended in power law fluids. Powder Technol. 2017, 320, 546–554. [Google Scholar] [CrossRef]
- Dubey, R.; Singh, G.; Majumder, A. Roping: Is it an optimum dewatering performance condition in a hydrocyclone? Powder Technol. 2017, 321, 218–231. [Google Scholar] [CrossRef]
- Patra, G.; Velpuri, B.; Chakraborty, S.; Meikap, B.C. Performance evaluation of a hydrocyclone with a spiral rib for separation of particles. Adv. Powder Technol. 2017, 28, 3222–3232. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, Q.; Zhang, B.; Tian, F. Three-phase hydrocyclone separator—A review. Chem. Eng. Res. Des. 2015, 100, 554–560. [Google Scholar] [CrossRef]
- Colli, A.N.; Fornés, J.P.; Pérez, O.G.; Bisang, J.M. Evaluation of a modified hydrocyclone as electrochemical reactor for processing of two-phase (gas-liquid) systems. Electrochim. Acta 2019, 309, 219–227. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Q.; Wang, C.-Y.; Ge, X.-L.; Wang, H.-L. Dissolved gas separation using the pressure drop and centrifugal characteristics of an inner cone hydrocyclone. Sep. Purif. Technol. 2016, 161, 121–128. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wu, R.-M. Experimental and simulation of a novel hydrocyclone-tubular membrane as overflow pipe. Sep. Purif. Technol. 2018, 198, 60–67. [Google Scholar] [CrossRef]






| Parameter | Value (mm) |
|---|---|
| Body diameter | 478 |
| Inlet diameter | 188 |
| Vortex-finder diameter | 230 |
| Spigot diameter | 202 |
| Vortex-finder length | 257 |
| Cylindrical-part length | 478 |
| Included angle | 20° |
| Lot Number | Pressure Differential MPa 1 | Overflow Port Carrier D50 μm | Underflow Port Carrier D50 μm |
|---|---|---|---|
| ZG-1-01 | 0.16 | 41.7 | 50.9 |
| 0.16 | 41.5 | 53.7 | |
| ZG-1-02 | 0.18 | 40.0 | 46.7 |
| 0.18 | 32.1 | 52.5 | |
| ZG-1-03 | 0.20 | 43.3 | 49.8 |
| 0.20 | 45.1 | 47.2 | |
| ZG-1-04 | 0.23 | 42.8 | 50.3 |
| 0.23 | 31.3 | 46.6 | |
| ZG-1-05 | 0.24 | 39.8 | 52.2 |
| ZG-1-06 | 0.28 | 37.7 | 53.1 |
| ZG-1-07 | 0.30 | 40.7 | 56.6 |
| 0.30 | 36.9 | 59.7 | |
| 0.30 | 40.0 | 50.0 |
| Test Batch | Carrier Concentration (wt%) | Pressure Difference 1 (MPa) | Overflow Port Carrier D50 μm | Underflow Port Carrier D50 μm |
|---|---|---|---|---|
| ZG-2-01 | 16.4 | 0.30 | 38.2 | 57.4 |
| 0.27 | 37.5 | 50.1 | ||
| 0.25 | 38.6 | 43.2 | ||
| ZG-2-02 | 14.9 | 0.30 | 39.4 | 60.9 |
| 0.27 | 38.7 | 56.9 | ||
| 0.25 | 32.2 | 55.6 | ||
| ZG-2-03 | 14.1 | 0.30 | 42.8 | 65.2 |
| 0.27 | 33.6 | 59.9 | ||
| 0.25 | 33.7 | 59.8 | ||
| ZG-2-04 | 13.0 | 0.30 | 39.5 | 61.9 |
| 0.27 | 43.4 | 61.8 | ||
| 0.25 | 40.9 | 63.5 |
| Test Batch | Sample | Overflow Port Carrier D50 μm | Underflow Port Carrier D50 μm |
|---|---|---|---|
| ZG-2-05 | Sample 1 | 40.8 | 63.0 |
| Sample 2 | 41.0 | 63.2 | |
| ZG-2-06 | Sample 1 | 42.2 | 62.9 |
| Sample 2 | 40.6 | 61.4 | |
| ZG-2-07 | Sample 1 | 40.6 | 62.1 |
| Sample 2 | 41.9 | 61.7 |
| Lot Number 1 | Pre-Separation Carrier D50 μm | Overflow Port Carrier D50 μm | Underflow Port Carrier D50 μm |
|---|---|---|---|
| ZG-3-01 | 36.8 | 33.7 | 51.5 |
| ZG-3-02 | 45.5 | 38.4 | 63.0 |
| ZG-3-03 | 46.7 | 36.8 | 65.1 |
| ZG-3-04 | 46.9 | 37.6 | 66.5 |
| ZG-3-05 | 47.1 | 35.5 | 65.0 |
| Lot Number | D10 μm | D50 μm | D90 μm | SPAN | Weight kg | Remark |
|---|---|---|---|---|---|---|
| CS-01 | 18.0 | 33.8 | 46.7 | 0.66 | 178.8 | Overflow port |
| 45.0 | 65.1 | 85.9 | 0.65 | 130.0 | Underflow port | |
| CS-02 | 19.7 | 31.6 | 42.2 | 0.70 | 171.8 | Overflow port |
| 48.4 | 66.5 | 90.8 | 0.69 | 129.6 | Underflow port | |
| CS-03 | 13.6 | 25.5 | 49.2 | 0.72 | 187.9 | Overflow port |
| 46.5 | 65.0 | 80.9 | 0.53 | 122.1 | Underflow port |
| Lot Number | D10 μm | D50 μm | D90 μm | SPAN | Weight kg | Remark |
|---|---|---|---|---|---|---|
| SG-01 | 41.5 | 50.9 | 84.2 | 0.73 | 138.9 | >250 |
| 23.8 | 36.8 | 57.4 | 0.91 | 186.4 | <250 | |
| SG-02 | 46.8 | 61.9 | 80.0 | 0.54 | 153.0 | >250 |
| 24.6 | 39.7 | 56.9 | 0.81 | 170.3 | <250 | |
| SG-03 | 45.4 | 63.7 | 87.3 | 0.66 | 159.7 | >250 |
| 29.8 | 41.0 | 56.0 | 0.64 | 173.1 | <250 |
| Sample Lot Number | D50 μm | Strength Factor L | Remark |
|---|---|---|---|
| CS-01 | 65.1 | 0.146 | Hydrocyclone separation |
| CS-03 | 65.0 | 0.152 | Hydrocyclone separation |
| SG-04 | 57.0 | 0.225 | Screening separation |
| SG-05 | 56.1 | 0.265 | Screening separation |
| Lot Number | Ti (%) | DIBP (%) | DEP (%) | Volatile | D10 | D50 | D90 | Span | Activity | BD |
|---|---|---|---|---|---|---|---|---|---|---|
| CP-01 | 2.28 | 8.19 | 1.16 | 13.06 | 40.1 | 67.5 | 93.5 | 0.68 | 6.77 | 0.475 |
| Imported-1 | 2.32 | 8.13 | 1.39 | 13.51 | 39.9 | 64.1 | 95.1 | 0.72 | 6.19 | 0.475 |
| Imported-2 | 2.28 | 7.98 | 1.35 | 13.48 | 41.3 | 66.9 | 96.9 | 0.70 | 6.04 | 0.471 |
| Numbering | Hypergravity Machine Speed (rpm) | Carrier Particle Size (μm) | SPAN | Alcohol-Magnesium Ratio 1 | ||
|---|---|---|---|---|---|---|
| D10 | D50 | D90 | ||||
| CP-1-01 | 2400 | 26.3 | 45.0 | 76.9 | 1.12 | 2.61 |
| CP-1-02 | 2000 | 30.3 | 50.3 | 84.2 | 1.07 | 2.62 |
| CP-1-03 | 1600 | 29.5 | 52.5 | 92.8 | 1.21 | 2.59 |
| CP-1-04 | 800 | 23.8 | 50.9 | 94.1 | 1.38 | 2.57 |
| CP-1-05 | 500 | 26.3 | 50.2 | 89.4 | 1.26 | 2.62 |
| Numbering | Screen Length (m) | Number of Screen Layers | Carrier Particle Size (μm) | SPAN | Alcohol-Magnesium Ratio | ||
|---|---|---|---|---|---|---|---|
| D10 | D50 | D90 | |||||
| CP-2-01 | 33 | 27 | 26.5 | 44.7 | 73.3 | 1.04 | 2.69 |
| CP-2-02 | 28 | 23 | 29.1 | 44.6 | 69.4 | 0.90 | 2.65 |
| CP-2-03 | 24 | 19 | 27.8 | 45.0 | 70.3 | 0.94 | 2.56 |
| CP-2-04 | 16 | 13 | 27.2 | 45.6 | 77.1 | 1.09 | 2.65 |
| CP-2-05 | 8 | 7 | 23.8 | 46.5 | 80.3 | 1.22 | 2.66 |
| Numbering | Melt the Kettle Speed (r/min) | Carrier Particle Size (μm) | SPAN | Alcohol-Magnesium Ratio | ||
|---|---|---|---|---|---|---|
| D10 | D50 | D90 | ||||
| CP-3-01 | 160 | 27.5 | 47.2 | 82.1 | 1.16 | 2.56 |
| CP-3-02 | 130 | 27.0 | 47.1 | 76.8 | 1.06 | 2.57 |
| CP-3-03 | 70 | 27.7 | 47.4 | 80.1 | 1.11 | 2.57 |
| CP-3-04 | 50 | 23.9 | 60.6 | 129.2 | 1.74 | 2.50 |
| Numbering | White Oil Volume (L) | Silicone Oil Volume (L) | Carrier Particle Size (μm). | SPAN | Alcohol-Magnesium Ratio | ||
|---|---|---|---|---|---|---|---|
| D10 | D50 | D90 | |||||
| CP-4-01 | 100 | 800 | 27.9 | 42.4 | 65.3 | 0.88 | 2.66 |
| CP-4-02 | 150 | 750 | 25.6 | 44.8 | 69.1 | 0.97 | 2.60 |
| CP-4-03 | 180 | 720 | 30.4 | 47.0 | 73.1 | 0.91 | 2.60 |
| CP-4-04 | 200 | 700 | 28.1 | 45.9 | 75.0 | 1.02 | 2.55 |
| CP-4-05 | 230 | 670 | 28.9 | 46.8 | 75.8 | 1.00 | 2.55 |
| CP-4-06 | 300 | 600 | 27.3 | 47.0 | 80.8 | 1.13 | 2.55 |
| CP-4-07 | 450 | 450 | 22.9 | 70.5 | 129.4 | 1.51 | 2.53 |
| CP-4-08 | 600 | 300 | 28.3 | 68.5 | 133.4 | 1.53 | 2.53 |
| CP-4-09 | 700 | 200 | 24.3 | 74.8 | 182.6 | 2.11 | 2.52 |
| Numbering | Magnesium Chloride Input (kg) | Carrier Particle Size (μm) | SPAN | Alcohol-Magnesium Ratio | ||
|---|---|---|---|---|---|---|
| D10 | D50 | D90 | ||||
| CP-5-01 | 36 | 25.8 | 40.2 | 64.4 | 0.96 | 2.66 |
| CP-5-02 | 65 | 24.3 | 40.2 | 62.1 | 0.94 | 2.63 |
| CP-5-03 | 78 | 31.7 | 47.2 | 71.1 | 0.84 | 2.61 |
| CP-5-04 | 86 | 31.5 | 49.8 | 80.3 | 0.98 | 2.56 |
| CP-5-05 | 98 | 35.0 | 52.3 | 78.7 | 0.84 | 2.52 |
| CP-5-06 | 106 | 35.9 | 56.5 | 88.4 | 0.93 | 2.50 |
| CP-5-07 | 120 | 37.5 | 63.0 | 108.8 | 1.13 | 2.50 |
| CP-5-08 | 140 | 44.6 | 72.0 | 117.8 | 1.02 | 2.54 |
| Numbering | Magnesium Chloride Input (kg) | Carrier Particle Size (μm) | SPAN | Alcohol-Magnesium Ratio | ||
|---|---|---|---|---|---|---|
| D10 | D50 | D90 | ||||
| CP-01 | 120 | 37.5 | 63.0 | 108.8 | 1.13 | 2.50 |
| CP-02 | 120 | 37.7 | 63.6 | 106.2 | 1.08 | 2.53 |
| CP-03 | 120 | 33.7 | 61.1 | 112.4 | 1.09 | 2.60 |
| CP-04 | 140 | 44.6 | 72.0 | 117.8 | 1.02 | 2.54 |
| CP-05 | 140 | 45.5 | 74.1 | 121.1 | 1.02 | 2.55 |
| Lot Number | Conditions | D10μm | D50μm | D90μm | SPAN | Weight (kg) | Yield |
|---|---|---|---|---|---|---|---|
| CP-06 | Before grading | 44.6 | 52.0 | 117.8 | 1.02 | 308.4 | |
| Overflow port | 19.4 | 32.4 | 58.8 | 0.74 | 166.5 | 54% | |
| Underflow port | 50.1 | 74.1 | 97.8 | 0.66 | 140.6 | 46% | |
| CP-07 | Before grading | 45.5 | 54.1 | 121.1 | 1.00 | 336.0 | |
| Overflow port | 25.1 | 41.7 | 61.9 | 0.83 | 188.7 | 56% | |
| Underflow port | 59.8 | 78.3 | 110.8 | 0.68 | 144.5 | 43% |
| Lot Number | Ti % | DIBP % | DEP % | Volatile % | D10 μm | D50 Μm | D90 μm | SPAN | Activity | BD | MI g/min 1 | II % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CP-01 | 3.12 | 11.04 | 0.27 | 12.13 | 55.3 | 73.2 | 95.1 | 0.64 | 6.40 | 0.468 | 27.9 | 97.6 |
| Goal | 50.0 | 71.0 | 96.0 | 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.; Du, Y.; Du, S.; Xiang, L. Graded Preparation and Industrial Applications of Large-Ball Polyolefin Catalyst Carriers. Catalysts 2022, 12, 117. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12020117
Lv X, Du Y, Du S, Xiang L. Graded Preparation and Industrial Applications of Large-Ball Polyolefin Catalyst Carriers. Catalysts. 2022; 12(2):117. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12020117
Chicago/Turabian StyleLv, Xinfeng, Yuhao Du, Shanjun Du, and Lan Xiang. 2022. "Graded Preparation and Industrial Applications of Large-Ball Polyolefin Catalyst Carriers" Catalysts 12, no. 2: 117. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12020117