Ubiquitin Regulation: The Histone Modifying Enzyme′s Story
Abstract
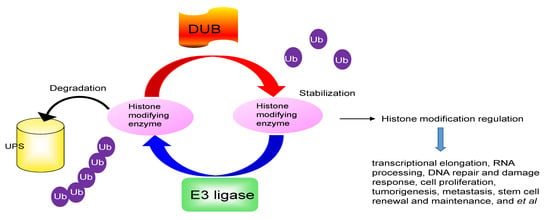
:1. Introduction
2. Ubiquitin Proteasomal Degradation of Histone Acetylation Enzymes
2.1. Histone Acetyltransferases
2.1.1. p300
2.1.2. PCAF (p300/CBP--Associated Factor)
2.1.3. HBO1 (Histone Acetyltransferase Binding to Origin Recognition Complex 1)
2.1.4. Tip60
2.2. Histone Deacetyltransferases
HDAC1/HDAC2
3. Ubiquitin Regulation of Histone Methylation Enzymes
3.1. Histone Methylation Enzymes
3.1.1. SETD2/SETD3 (The SET-Domain Methyltransferase 2/3)
3.1.2. PR-Set7/Set8 (The SET-Domain Methyltransferase PR-Set7)
3.1.3. EZH2 (Enhancer of Zeste Homolog 2)
3.2. Histone Demethylation Enzymes
3.2.1. JMJD2A (Jumonji Domain 2)
3.2.2. LSD1 (Lysine-Specific Demethylase 1)
4. Ubiquitin of Histone Arginine Methylation Enzymes
4.1. PRMT1
4.2. PRMT4
5. Ubiquitination of Histone Modification Readers
BRD4 (Bromodomain Containing 4)
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bernstein, B.E.; Meissner, A.; Lander, E.S. The mammalian epigenome. Cell 2007, 128, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, R.D.; Lorch, Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell 1999, 98, 285–294. [Google Scholar] [CrossRef]
- Richmond, T.J.; Davey, C.A. The structure of DNA in the nucleosome core. Nature 2003, 423, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Luger, K.; Richmond, T.J. The histone tails of the nucleosome. Curr. Opin. Genet. Dev. 1998, 8, 140–146. [Google Scholar] [CrossRef]
- Tessarz, P.; Kouzarides, T. Histone core modifications regulating nucleosome structure and dynamics. Nat. Rev. Mol. Cell Biol. 2014, 15, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Jenuwein, T.; Allis, C.D. Translating the histone code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Pickart, C.M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 2001, 70, 503–533. [Google Scholar] [CrossRef] [PubMed]
- Haas, A.L.; Rose, I.A. The mechanism of ubiquitin activating enzyme. A kinetic and equilibrium analysis. J. Biol. Chem. 1982, 257, 10329–10337. [Google Scholar] [PubMed]
- Scheffner, M.; Nuber, U.; Huibregtse, J.M. Protein ubiquitination involving an E1-E2-E3 enzyme ubiquitin thioester cascade. Nature 1995, 373, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Kim, N.G.; Gumbiner, B.M. Regulation of protein stability by GSK3 mediated phosphorylation. Cell Cycle 2009, 8, 4032–4039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, S.H.; Mohawk, J.A.; Siepka, S.M.; Shan, Y.; Huh, S.K.; Hong, H.K.; Kornblum, I.; Kumar, V.; Koike, N.; Xu, M.; et al. Competing E3 ubiquitin ligases govern circadian periodicity by degradation of CRY in nucleus and cytoplasm. Cell 2013, 152, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Ravid, T.; Hochstrasser, M. Diversity of degradation signals in the ubiquitin-proteasome system. Nat. Rev. Mol. Cell Biol. 2008, 9, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K.D. Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J. 1997, 11, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Fraile, J.M.; Quesada, V.; Rodriguez, D.; Freije, J.M.; Lopez-Otin, C. Deubiquitinases in cancer: New functions and therapeutic options. Oncogene 2012, 31, 2373–2388. [Google Scholar] [CrossRef] [PubMed]
- Hanpude, P.; Bhattacharya, S.; Dey, A.K.; Maiti, T.K. Deubiquitinating enzymes in cellular signaling and disease regulation. IUBMB Life 2015, 67, 544–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valor, L.M.; Viosca, J.; Lopez-Atalaya, J.P.; Barco, A. Lysine acetyltransferases CBP and p300 as therapeutic targets in cognitive and neurodegenerative disorders. Curr. Pharm. Des. 2013, 19, 5051–5064. [Google Scholar] [CrossRef] [PubMed]
- Ogryzko, V.V.; Schiltz, R.L.; Russanova, V.; Howard, B.H.; Nakatani, Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 1996, 87, 953–959. [Google Scholar] [CrossRef]
- Goodman, R.H.; Smolik, S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000, 14, 1553–1577. [Google Scholar] [PubMed]
- Iyer, N.G.; Ozdag, H.; Caldas, C. P300/CBP and cancer. Oncogene 2004, 23, 4225–4231. [Google Scholar] [CrossRef] [PubMed]
- Poizat, C.; Sartorelli, V.; Chung, G.; Kloner, R.A.; Kedes, L. Proteasome-mediated degradation of the coactivator p300 impairs cardiac transcription. Mol. Cell Biol. 2000, 20, 8643–8654. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Molina, S.; Oliva, J.L.; Garcia-Vargas, S.; Valls, E.; Rojas, J.M.; Martinez-Balbas, M.A. The histone acetyltransferases CBP/p300 are degraded in NIH 3T3 cells by activation of Ras signalling pathway. Biochem. J. 2006, 398, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.E.; Han, C.; Zhao, R.; Wani, G.; Zhu, Q.; Gong, L.; Battu, A.; Racoma, I.; Sharma, N.; Wani, A.A. P38 MAPK- and Akt-mediated p300 phosphorylation regulates its degradation to facilitate nucleotide excision repair. Nucleic Acids Res. 2013, 41, 1722–1733. [Google Scholar] [CrossRef] [PubMed]
- Shima, Y.; Shima, T.; Chiba, T.; Irimura, T.; Pandolfi, P.P.; Kitabayashi, I. PML activates transcription by protecting HIPK2 and p300 from SCFFbx3-mediated degradation. Mol. Cell Biol. 2008, 28, 7126–7138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mayo, M.W.; Nagji, A.S.; Hall, E.H.; Shock, L.S.; Xiao, A.; Stelow, E.B.; Jones, D.R. BRMS1 suppresses lung cancer metastases through an E3 ligase function on histone acetyltransferase p300. Cancer Res. 2013, 73, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.; Schon, M.; Bayerlova, M.; Bleckmann, A.; Schon, M.P.; Zornig, M.; Dobbelstein, M. A pro-apoptotic function of iASPP by stabilizing p300 and CBP through inhibition of BRMS1 E3 ubiquitin ligase activity. Cell Death Dis. 2015, 6, e1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiltz, R.L.; Nakatani, Y. The PCAF acetylase complex as a potential tumor suppressor. Biochim. Biophys. Acta 2000, 1470, M37–M53. [Google Scholar] [CrossRef]
- Blanco, J.C.; Minucci, S.; Lu, J.; Yang, X.J.; Walker, K.K.; Chen, H.; Evans, R.M.; Nakatani, Y.; Ozato, K. The histone acetylase PCAF is a nuclear receptor coactivator. Genes Dev. 1998, 12, 1638–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Zeng, S.X.; Lee, H.; Lu, H. MDM2 mediates p300/CREB-binding protein-associated factor ubiquitination and degradation. J. Biol. Chem. 2004, 279, 20035–20043. [Google Scholar] [CrossRef] [PubMed]
- Linares, L.K.; Kiernan, R.; Triboulet, R.; Chable-Bessia, C.; Latreille, D.; Cuvier, O.; Lacroix, M.; Le Cam, L.; Coux, O.; Benkirane, M. Intrinsic ubiquitination activity of PCAF controls the stability of the oncoprotein Hdm2. Nat. Cell Biol. 2007, 9, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Mazza, D.; Infante, P.; Colicchia, V.; Greco, A.; Alfonsi, R.; Siler, M.; Antonucci, L.; Po, A.; De Smaele, E.; Ferretti, E.; et al. PCAF ubiquitin ligase activity inhibits Hedgehog/Gli1 signaling in p53-dependent response to genotoxic stress. Cell Death Differ. 2013, 20, 1688–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.L.; Yu, S.Y.; Li, M. Functional analysis of HBO1 in tumor development and inhibitor screening. Int. J. Mol. Med. 2016, 38, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Chen, Y.; Smith, R.M.; Snavely, C.; Li, J.; Coon, T.A.; Chen, B.B.; Zhao, Y.; Mallampalli, R.K. SCFFfbxw15 mediates histone acetyltransferase binding to origin recognition complex (HBO1) ubiquitin-proteasomal degradation to regulate cell proliferation. J. Biol. Chem. 2013, 288, 6306–6316. [Google Scholar] [CrossRef] [PubMed]
- Matsunuma, R.; Niida, H.; Ohhata, T.; Kitagawa, K.; Sakai, S.; Uchida, C.; Shiotani, B.; Matsumoto, M.; Nakayama, K.I.; Ogura, H.; et al. UV damage-induced phosphorylation of HBO1 triggers CRL4DDB2-mediated degradation to regulate cell proliferation. Mol. Cell Biol. 2016, 36, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, M.; Susa, T.; Takahashi, Y.; Tamamori-Adachi, M.; Kajitani, T.; Okinaga, H.; Fukusato, T.; Okazaki, T. Histone acetyltransferase HBO1 destabilizes estrogen receptor alpha by ubiquitination and modulates proliferation of breast cancers. Cancer Sci. 2013, 104, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, M.; Susa, T.; Tamamori-Adachi, M.; Okinaga, H.; Okazaki, T. Intrinsic ubiquitin E3 ligase activity of histone acetyltransferase Hbo1 for estrogen receptor α. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 498–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Judes, G.; Rifai, K.; Ngollo, M.; Daures, M.; Bignon, Y.J.; Penault-Llorca, F.; Bernard-Gallon, D. A bivalent role of TIP60 histone acetyl transferase in human cancer. Epigenomics 2015, 7, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Achour, M.; Fuhrmann, G.; Alhosin, M.; Ronde, P.; Chataigneau, T.; Mousli, M.; Schini-Kerth, V.B.; Bronner, C. UHRF1 recruits the histone acetyltransferase Tip60 and controls its expression and activity. Biochem. Biophys. Res. Commun. 2009, 390, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Wang, Y.; Zhang, T.; Bai, L.; Wang, Y.; Duan, C. E3 ligase UHRF2 stabilizes the acetyltransferase TIP60 and regulates H3K9ac and H3K14ac via RING finger domain. Protein Cell 2017, 8, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Bhoumik, A.; Singha, N.; O’Connell, M.J.; Ronai, Z.A. Regulation of TIP60 by ATF2 modulates ATM activation. J. Biol. Chem. 2008, 283, 17605–17614. [Google Scholar] [CrossRef] [PubMed]
- Legube, G.; Linares, L.K.; Lemercier, C.; Scheffner, M.; Khochbin, S.; Trouche, D. Tip60 is targeted to proteasome-mediated degradation by Mdm2 and accumulates after UV irradiation. EMBO J. 2002, 21, 1704–1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbaiah, V.K.; Zhang, Y.; Rajagopalan, D.; Abdullah, L.N.; Yeo-Teh, N.S.; Tomaic, V.; Banks, L.; Myers, M.P.; Chow, E.K.; Jha, S. E3 ligase EDD1/UBR5 is utilized by the HPV E6 oncogene to destabilize tumor suppressor tip60. Oncogene 2016, 35, 2062–2074. [Google Scholar] [CrossRef] [PubMed]
- Tam, L.M.; Jiang, J.; Wang, P.; Li, L.; Miao, W.; Dong, X.; Wang, Y. Arsenite binds to the zinc finger motif of TIP60 histone acetyltransferase and induces its degradation via the 26s proteasome. Chem. Res. Toxicol. 2017, 30, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, L.; Wu, J.; Sokirniy, I.; Nguyen, P.; Bregnard, T.; Weinstock, J.; Mattern, M.; Bezsonova, I.; Hancock, W.W.; et al. Active site-targeted covalent irreversible inhibitors of USP7 impair the functions of Foxp3+ T-regulatory cells by promoting ubiquitination of Tip60. PloS ONE 2017, 12, e0189744. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kumar, S.; Dahiya, S.; Wang, F.; Wu, J.; Newick, K.; Han, R.; Samanta, A.; Beier, U.H.; Akimova, T.; et al. Ubiquitin-specific protease-7 inhibition impairs Tip60-dependent Foxp3+ T-regulatory cell function and promotes antitumor immunity. EBioMedicine 2016, 13, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.; Shibata, E.; Dutta, A. Deubiquitination of Tip60 by USP7 determines the activity of the p53-dependent apoptotic pathway. Mol. Cell Biol. 2013, 33, 3309–3320. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Koppen, A.; Rakhshandehroo, M.; Tasdelen, I.; van de Graaf, S.F.; van Loosdregt, J.; van Beekum, O.; Hamers, N.; van Leenen, D.; Berkers, C.R.; et al. Early adipogenesis is regulated through USP7-mediated deubiquitination of the histone acetyltransferase Tip60. Nat. Commun. 2013, 4, 2656. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Guo, M.; Xu, D.; Ding, Z.C.; Zhou, G.; Ding, H.F.; Zhang, J.; Tang, Y.; Yan, C. The stress-responsive gene ATF3 regulates the histone acetyltransferase Tip60. Nat. Commun. 2015, 6, 6752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiper-Bergeron, N.; Wu, D.; Pope, L.; Schild-Poulter, C.; Hache, R.J. Stimulation of preadipocyte differentiation by steroid through targeting of an HDAC1 complex. EMBO J. 2003, 22, 2135–2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaughan, L.; Logan, I.R.; Neal, D.E.; Robson, C.N. Regulation of androgen receptor and histone deacetylase 1 by Mdm2-mediated ubiquitylation. Nucleic Acids Res. 2005, 33, 13–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, D.H.; Eom, G.H.; Ko, J.H.; Shin, S.; Joung, H.; Choe, N.; Nam, Y.S.; Min, H.K.; Kook, T.; Yoon, S.; et al. Mdm2 E3 ligase-mediated ubiquitination and degradation of HDAC1 in vascular calcification. Nat. Commun. 2016, 7, 10492. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.M.; Kwon, Y.E.; Kim, J.M.; Bae, S.J.; Lee, B.K.; Yoo, S.J.; Chung, C.H.; Deshaies, R.J.; Seol, J.H. Chfr is linked to tumour metastasis through the downregulation of HDAC1. Nat. Cell Biol. 2009, 11, 295–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canettieri, G.; Di Marcotullio, L.; Greco, A.; Coni, S.; Antonucci, L.; Infante, P.; Pietrosanti, L.; De Smaele, E.; Ferretti, E.; Miele, E.; et al. Histone deacetylase and Cullin3-RENKCTD11 ubiquitin ligase interplay regulates hedgehog signalling through Gli acetylation. Nat. Cell Biol. 2010, 12, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Kramer, O.H.; Zhu, P.; Ostendorff, H.P.; Golebiewski, M.; Tiefenbach, J.; Peters, M.A.; Brill, B.; Groner, B.; Bach, I.; Heinzel, T.; et al. The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. EMBO J. 2003, 22, 3411–3420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Kan, S.; Huang, B.; Hao, Z.; Mak, T.W.; Zhong, Q. Mule determines the apoptotic response to HDAC inhibitors by targeted ubiquitination and destruction of HDAC2. Genes Dev. 2011, 25, 2610–2618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adenuga, D.; Yao, H.; March, T.H.; Seagrave, J.; Rahman, I. Histone deacetylase 2 is phosphorylated, ubiquitinated, and degraded by cigarette smoke. Am. J. Respir. Cell Mol. Biol. 2009, 40, 464–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Hao, Q.; Luo, J.; Xiong, J.; Zhang, S.; Wang, T.; Bai, L.; Wang, W.; Chen, M.; Wang, W.; et al. USP4 inhibits p53 and NF-κB through deubiquitinating and stabilizing HDAC2. Oncogene 2016, 35, 2902–2912. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Tao, L.; Chen, C.; Pan, L.; Hao, J.; Ni, Y.; Li, D.; Li, B.; Shi, G. USP17-mediated deubiquitination and stabilization of HDAC2 in cigarette smoke extract-induced inflammation. Int. J. Clin. Exp. Pathol. 2015, 8, 10707–10715. [Google Scholar] [PubMed]
- Li, J.; Duns, G.; Westers, H.; Sijmons, R.; van den Berg, A.; Kok, K. SETD2: An epigenetic modifier with tumor suppressor functionality. Oncotarget 2016, 7, 50719–50734. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, S.L.; Strahl, B.D. Shaping the cellular landscape with Set2/SETD2 methylation. Cell. Mol. Life Sci. CMLS 2017, 74, 3317–3334. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.M.; Kizer, K.O.; Braberg, H.; Krogan, N.J.; Strahl, B.D. RNA polymerase ii carboxyl-terminal domain phosphorylation regulates protein stability of the Set2 methyltransferase and histone H3 di- and trimethylation at lysine 36. J. Biol. Chem. 2012, 287, 3249–3256. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Lei, P.J.; Ju, L.G.; Wang, X.; Huang, K.; Yang, B.; Shao, C.; Zhu, Y.; Wei, G.; Fu, X.D.; et al. SPOP-containing complex regulates SETD2 stability and H3K36me3-coupled alternative splicing. Nucleic Acids Res. 2017, 45, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Hao, Y.; Shu, W.; Zhao, M.; Zhao, C.; Wu, Y.; Peng, X.; Yao, P.; Xiao, D.; Qing, G.; et al. Cell cycle-dependent degradation of the methyltransferase SETD3 attenuates cell proliferation and liver tumorigenesis. J. Biol. Chem. 2017, 292, 9022–9033. [Google Scholar] [CrossRef] [PubMed]
- Beck, D.B.; Oda, H.; Shen, S.S.; Reinberg, D. PR-Set7 and H4K20me1: At the crossroads of genome integrity, cell cycle, chromosome condensation, and transcription. Genes Dev. 2012, 26, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Yu, V.C.; Zhu, G.; Chang, D.C. SET8 plays a role in controlling G1/S transition by blocking lysine acetylation in histone through binding to H4 N-terminal tail. Cell Cycle 2008, 7, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Tardat, M.; Brustel, J.; Kirsh, O.; Lefevbre, C.; Callanan, M.; Sardet, C.; Julien, E. The histone H4 Lys 20 methyltransferase PR-Set7 regulates replication origins in mammalian cells. Nat. Cell Biol. 2010, 12, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Centore, R.C.; Havens, C.G.; Manning, A.L.; Li, J.M.; Flynn, R.L.; Tse, A.; Jin, J.; Dyson, N.J.; Walter, J.C.; Zou, L. CRL4Cdt2-mediated destruction of the histone methyltransferase Set8 prevents premature chromatin compaction in S phase. Mol. Cell 2010, 40, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Oda, H.; Hubner, M.R.; Beck, D.B.; Vermeulen, M.; Hurwitz, J.; Spector, D.L.; Reinberg, D. Regulation of the histone H4 monomethylase PR-Set7 by CRL4Cdt2-mediated PCNA-dependent degradation during DNA damage. Mol. Cell 2010, 40, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Shibata, E.; Park, J.; Jha, S.; Karnani, N.; Dutta, A. CRL4Cdt2 regulates cell proliferation and histone gene expression by targeting PR-Set7/Set8 for degradation. Mol. Cell 2010, 40, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, S.; Eskildsen, M.; Fugger, K.; Hansen, L.; Larsen, M.S.; Kousholt, A.N.; Syljuasen, R.G.; Trelle, M.B.; Jensen, O.N.; Helin, K.; et al. SET8 is degraded via PCNA-coupled CRL4(CDT2) ubiquitylation in S phase and after UV irradiation. J. Cell Biol. 2011, 192, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Wang, W.; Kong, X.; Congdon, L.M.; Yokomori, K.; Kirschner, M.W.; Rice, J.C. Dynamic regulation of the PR-Set7 histone methyltransferase is required for normal cell cycle progression. Genes Dev. 2010, 24, 2531–2542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Dai, X.; Zhong, J.; Inuzuka, H.; Wan, L.; Li, X.; Wang, L.; Ye, X.; Sun, L.; Gao, D.; et al. SCFβ-TRCP promotes cell growth by targeting PR-Set7/Set8 for degradation. Nat. Commun. 2015, 6, 10185. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Dai, X.; Wang, Z.; Wei, W. A new layer of degradation mechanism forPR-Set7/Set8 during cell cycle. Cell Cycle 2016, 15, 3042–3047. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Hart, C.M.; Francis, N.J.; Vargas, M.L.; Sengupta, A.; Wild, B.; Miller, E.L.; O’Connor, M.B.; Kingston, R.E.; Simon, J.A. Histone methyltransferase activity of a drosophila polycomb group repressor complex. Cell 2002, 111, 197–208. [Google Scholar] [CrossRef]
- Yan, K.S.; Lin, C.Y.; Liao, T.W.; Peng, C.M.; Lee, S.C.; Liu, Y.J.; Chan, W.P.; Chou, R.H. EZH2 in cancer progression and potential application in cancer therapy: A friend or foe? Int. J. Mol. Sci. 2017, 18, 1172. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.A.; Varambally, S.; Arend, R.C. Histone methyltransferase EZH2: A therapeutic target for ovarian cancer. Mol. Cancer Ther. 2018, 17, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.L.; Chou, R.H.; Shyu, W.C.; Hsieh, S.C.; Wu, C.S.; Chiang, S.Y.; Chang, W.J.; Chen, J.N.; Tseng, Y.J.; Lin, Y.H.; et al. Smurf2-mediated degradation of EZH2 enhances neuron differentiation and improves functional recovery after ischaemic stroke. EMBO Mol. Med. 2013, 5, 531–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahasrabuddhe, A.A.; Chen, X.; Chung, F.; Velusamy, T.; Lim, M.S.; Elenitoba-Johnson, K.S. Oncogenic Y641 mutations in EZH2 prevent Jak2/β-TrCp-mediated degradation. Oncogene 2015, 34, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yang, C.; Fan, P.; Xiao, J.; Zhang, W.; Zhan, S.; Liu, T.; Wang, D.; Wu, H. CDK5/FBW7-dependent ubiquitination and degradation of EZH2 inhibits pancreatic cancer cell migration and invasion. J. Biol. Chem. 2017, 292, 6269–6280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Consalvi, S.; Brancaccio, A.; Dall’Agnese, A.; Puri, P.L.; Palacios, D. Praja1 E3 ubiquitin ligase promotes skeletal myogenesis through degradation of EZH2 upon p38α activation. Nat. commun. 2017, 8, 13956. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Chen, L.; Yang, X.; Zhao, Y.; Pier, E.; Zhang, X.; Yang, X.; Xiong, Y. Downregulation of Ezh2 methyltransferase by FOXP3: New insight of FOXP3 into chromatin remodeling? Biochim. Biophys. Acta 2013, 1833, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, W.; Zhang, J.; Yan, M.; Xu, Q.; Wu, X.; Wan, L.; Zhang, Z.; Zhang, C.; Qin, X.; et al. A covalently bound inhibitor triggers EZH2 degradation through chip-mediated ubiquitination. EMBO J. 2017, 36, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hou, P.; Fan, D.; Dong, M.; Ma, M.; Li, H.; Yao, R.; Li, Y.; Wang, G.; Geng, P.; et al. The degradation of EZH2 mediated by IncRNA ANCR attenuated the invasion and metastasis of breast cancer. Cell Death Differ. 2017, 24, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, B.; Chen, D. USP21 promotes cell proliferation and metastasis through suppressing EZH2 ubiquitination in bladder carcinoma. Onco. Targets Ther. 2017, 10, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xiao, Z.; Wang, S.; Zhang, M.; Wei, Y.; Hang, Q.; Kim, J.; Yao, F.; Rodriguez-Aguayo, C.; Ton, B.N.; et al. ZRANB1 is an EZH2 deubiquitinase and a potential therapeutic target in breast cancer. Cell Rep. 2018, 23, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Berry, W.L.; Janknecht, R. Kdm4/jmjd2 histone demethylases: Epigenetic regulators in cancer cells. Cancer Res. 2013, 73, 2936–2942. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.S.; Kavanagh, K.L.; McDonough, M.A.; Butler, D.; Pilka, E.S.; Lienard, B.M.; Bray, J.E.; Savitsky, P.; Gileadi, O.; von Delft, F.; et al. Crystal structures of histone demethylase JMJD2a reveal basis for substrate specificity. Nature 2007, 448, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Calderas, L.; Gonzalez-Barrios, R.; Herrera, L.A.; Cantu de Leon, D.; Soto-Reyes, E. The role of the histone demethylase KDM4A in cancer. Cancer Genet. 2015, 208, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Van Rechem, C.; Black, J.C.; Abbas, T.; Allen, A.; Rinehart, C.A.; Yuan, G.C.; Dutta, A.; Whetstine, J.R. The SKP1-Cul1-F-box and leucine-rich repeat protein 4 (SCF-FbxL4) ubiquitin ligase regulates lysine demethylase 4A (KDM4A)/jumonji domain-containing 2A (JMJD2A) protein. J. Biol. Chem. 2011, 286, 30462–30470. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.K.; Lim, H.J.; Harper, J.W. SCFFBXO22 regulates histone H3 lysine 9 and 36 methylation levels by targeting histone demethylase KDM4A for ubiquitin-mediated proteasomal degradation. Mol. Cell Biol. 2011, 31, 3687–3699. [Google Scholar] [CrossRef] [PubMed]
- Mallette, F.A.; Mattiroli, F.; Cui, G.; Young, L.C.; Hendzel, M.J.; Mer, G.; Sixma, T.K.; Richard, S. RNF8- and RNF168-dependent degradation of KDM4AJMJD2A triggers 53BP1 recruitment to DNA damage sites. EMBO J. 2012, 31, 1865–1878. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.D.; Allis, C.D.; Bernstein, E. Epigenetics: A landscape takes shape. Cell 2007, 128, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lan, F.; Matson, C.; Mulligan, P.; Whetstine, J.R.; Cole, P.A.; Casero, R.A.; Shi, Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 2004, 119, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Schulte, J.H.; Lim, S.; Schramm, A.; Friedrichs, N.; Koster, J.; Versteeg, R.; Ora, I.; Pajtler, K.; Klein-Hitpass, L.; Kuhfittig-Kulle, S.; et al. Lysine-specific demethylase 1 is strongly expressed in poorly differentiated neuroblastoma: Implications for therapy. Cancer Res. 2009, 69, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Lin, K.; Zhang, S.; Chen, Y.; Zhang, N.; Xue, J.; Wang, Z.; Aldape, K.D.; Xie, K.; Woodgett, J.R.; et al. Nuclear GSK3β promotes tumorigenesis by phosphorylating KDM1A and inducing its deubiquitylation by USP22. Nat. Cell Biol. 2016, 18, 954–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozono, D.; Li, J.; Nitta, M.; Sampetrean, O.; Gonda, D.; Kushwaha, D.S.; Merzon, D.; Ramakrishnan, V.; Zhu, S.; Zhu, K.; et al. Dynamic epigenetic regulation of glioblastoma tumorigenicity through LSD1 modulation of myc expression. Proc. Natl. Acad. Sci. USA 2015, 112, E4055–E4064. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Park, J.H.; Park, M.; Won, H.Y.; Joo, H.S.; Lee, C.H.; Lee, J.Y.; Kong, G. UTX inhibits EMT-induced breast CSC properties by epigenetic repression of EMT genes in cooperation with LSD1 and HDAC1. EMBO Rep. 2015, 16, 1288–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayami, S.; Kelly, J.D.; Cho, H.S.; Yoshimatsu, M.; Unoki, M.; Tsunoda, T.; Field, H.I.; Neal, D.E.; Yamaue, H.; Ponder, B.A.; et al. Overexpression of LSD1 contributes to human carcinogenesis through chromatin regulation in various cancers. Int. J. Cancer 2011, 128, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Janzer, A.; Becker, A.; Zimmer, A.; Schule, R.; Buettner, R.; Kirfel, J. Lysine-specific demethylase 1 (LSD1) is highly expressed in ER-negative breast cancers and a biomarker predicting aggressive biology. Carcinogenesis 2010, 31, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, Z.M.; Xia, Y.; Liao, G.Q.; Pan, Y.; Liu, S.; Zhang, Y.; Yan, Z.S. LSD1-mediated epigenetic modification contributes to proliferation and metastasis of colon cancer. Br. J. Cancer 2013, 109, 994–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Ding, J.; Wang, Z.; Zhu, J.; Wang, X.; Du, J. Identification of downstream metastasis-associated target genes regulated by LSD1 in colon cancer cells. Oncotarget 2017, 8, 19609–19630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Xu, G.; Liu, J.; Zhang, N.; Li, L.; Ji, J.; Zhang, J.; Zhang, L.; Wang, G.; Wang, X.; et al. Phosphorylation of LSD1 at Ser112 is crucial for its function in induction of EMT and metastasis in breast cancer. Breast Cancer Res. Treat. 2016, 159, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, B.P. Epigenetic regulation of LSD1 during mammary carcinogenesis. Mol. Cell Oncol. 2014, 1, e963426. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, S.; Sacca, C.D.; Majello, B. Epigenetic regulation of epithelial to mesenchymal transition by the lysine-specific demethylase LSD1/KMD1A. Biochim. Biophys. Acta 2017, 1860, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wu, Y.; Li, J.; Dong, C.; Ye, X.; Chi, Y.I.; Evers, B.M.; Zhou, B.P. The SNAG domain of Snail1 functions as a molecular hook for recruiting lysine-specific demethylase 1. EMBO J. 2010, 29, 1803–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.J.; Matson, C.; Lan, F.; Iwase, S.; Baba, T.; Shi, Y. Regulation of LSD1 histone demethylase activity by its associated factors. Mol. Cell 2005, 19, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Yang, X.H.; Kang, T.; Zhao, Y.; Wang, C.; Evers, B.M.; Zhou, B.P. The deubiquitinase USP28 stabilizes LSD1 and confers stem-cell-like traits to breast cancer cells. Cell Rep. 2013, 5, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Cui, Y.; Xu, Q.; Jiang, Y. Stabilization of LSD1 by deubiquitinating enzyme USP7 promotes glioblastoma cell tumorigenesis and metastasis through suppression of the p53 signaling pathway. Oncol. Rep. 2016, 36, 2935–2945. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gui, B.; Xiong, C.; Zhao, L.; Liang, J.; Sun, L.; Yang, X.; Yu, W.; Si, W.; Yan, R.; et al. Destabilizing LSD1 by Jade-2 promotes neurogenesis: An antibraking system in neural development. Mol. Cell 2014, 55, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Panchenko, M.V. Structure, function and regulation of Jade family PHD finger 1 (JADE1). Gene 2016, 589, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.B.; Mallampalli, R.K. F-box protein substrate recognition: A new insight. Cell Cycle 2013, 12, 1009–1010. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Li, J.; Li, X.; Zou, C. LPS modulates p300 and Sirt1 to promote PRMT1 stability via an SCF-Fbxl17-recognized acetyldegron. J. Cell Sci. 2017, 130, 3578–3587. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Katagiri, K.; Nagaoka, K.; Morishita, T.; Kudoh, Y.; Hatta, T.; Naguro, I.; Kano, K.; Udagawa, T.; Natsume, T.; et al. TRIM48 promotes ASK1 activation and cell death through ubiquitination-dependent degradation of the ASK1-negative regulator prmt1. Cell Rep. 2017, 21, 2447–2457. [Google Scholar] [CrossRef] [PubMed]
- Bhuripanyo, K.; Wang, Y.; Liu, X.; Zhou, L.; Liu, R.; Duong, D.; Zhao, B.; Bi, Y.; Zhou, H.; Chen, G.; et al. Identifying the substrate proteins of U-box E3s E4B and CHIP by orthogonal ubiquitin transfer. Sci. Adv. 2018, 4, e1701393. [Google Scholar] [CrossRef] [PubMed]
- Cha, B.; Park, Y.; Hwang, B.N.; Kim, S.Y.; Jho, E.H. Protein arginine methyltransferase 1 methylates Smurf2. Mol. Cells 2015, 38, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Tikhanovich, I.; Kuravi, S.; Artigues, A.; Villar, M.T.; Dorko, K.; Nawabi, A.; Roberts, B.; Weinman, S.A. Dynamic arginine methylation of tumor necrosis factor (TNF) receptor-associated factor 6 regulates toll-like receptor signaling. J. Biol. Chem. 2015, 290, 22236–22249. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, Z.; Meyer, M.B.; Saha, S.; Yu, M.; Guo, A.; Wisinski, K.B.; Huang, W.; Cai, W.; Pike, J.W.; et al. CARM1 methylates chromatin remodeling factor BAF155 to enhance tumor progression and metastasis. Cancer Cell 2014, 25, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Habashy, H.O.; Rakha, E.A.; Ellis, I.O.; Powe, D.G. The oestrogen receptor coactivator CARM1 has an oncogenic effect and is associated with poor prognosis in breast cancer. Breast Cancer Res. Treat. 2013, 140, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Kim, H.; Oh, S.; Lee, J.G.; Kee, M.; Ko, H.J.; Kweon, M.N.; Won, K.J.; Baek, S.H. AMPK-SKP2-CARM1 signalling cascade in transcriptional regulation of autophagy. Nature 2016, 534, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, L.; Xue, H.; Yang, Z.; Yin, Y.; Zhang, B.; Chen, M.; Ma, H. Nuclear AMPK regulated CARM1 stabilization impacts autophagy in aged heart. Biochem. Biophys. Res. Commun. 2017, 486, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lim, S.; Park, M.; Choi, J.; Kim, J.; Han, H.; Yoon, K.; Kim, K.; Lim, J.; Park, S. Ubiquitination-dependent CARM1 degradation facilitates Notch1-mediated podocyte apoptosis in diabetic nephropathy. Cell. Signal. 2014, 26, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lai, Y.; Li, J.; Zou, M.; Zou, C. Oxidative stress destabilizes protein arginine methyltransferase 4 via glycogen synthase kinase 3β to impede lung epithelial cell migration. Am. J. Phys. Cell Phys. 2017, 313, C285–C294. [Google Scholar] [CrossRef] [PubMed]
- Janzen, W.P.; Wigle, T.J.; Jin, J.; Frye, S.V. Epigenetics: Tools and technologies. Drug Discov. Today Technol. 2010, 7, e59–e65. [Google Scholar] [CrossRef] [PubMed]
- Filippakopoulos, P.; Knapp, S. Targeting bromodomains: Epigenetic readers of lysine acetylation. Nat. Rev. Drug Discov. 2014, 13, 337–356. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Chitsaz, F.; Abbasi, A.; Misteli, T.; Ozato, K. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc. Natl. Acad. Sci. USA 2003, 100, 8758–8763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Gan, W.; Li, X.; Wang, S.; Zhang, W.; Huang, L.; Liu, S.; Zhong, Q.; Guo, J.; Zhang, J.; et al. Prostate cancer-associated SPOP mutations confer resistance to BET inhibitors through stabilization of BRD4. Nat. Med. 2017, 23, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Filippakopoulos, P. Beating the odds: BETs in disease. Trends Biochem. Sci. 2015, 40, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Y.; Zeng, L.; Wu, Y.; Deng, J.; Zhang, Q.; Lin, Y.; Li, J.; Kang, T.; Tao, M.; et al. Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like breast cancer. Cancer Cell 2014, 25, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, D.; Zhao, Y.; Ren, S.; Gao, K.; Ye, Z.; Wang, S.; Pan, C.W.; Zhu, Y.; Yan, Y.; et al. Intrinsic BET inhibitor resistance in SPOP-mutated prostate cancer is mediated by BET protein stabilization and AKT-mTORC1 activation. Nat. Med. 2017, 23, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Janouskova, H.; El Tekle, G.; Bellini, E.; Udeshi, N.D.; Rinaldi, A.; Ulbricht, A.; Bernasocchi, T.; Civenni, G.; Losa, M.; Svinkina, T.; et al. Opposing effects of cancer-type-specific SPOP mutants on BET protein degradation and sensitivity to BET inhibitors. Nat. Med. 2017, 23, 1046–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akuffo, A.A.; Alontaga, A.Y.; Metcalf, R.; Beatty, M.S.; Becker, A.; McDaniel, J.M.; Hesterberg, R.S.; Goodheart, W.E.; Gunawan, S.; Ayaz, M.; et al. Ligand-mediated protein degradation reveals functional conservation among sequence variants of the CUL4-type E3 ligase substrate receptor cereblon. J. Biol. Chem. 2018, 293, 6187–6200. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yan, Y.; Wang, D.; Ding, D.; Ma, T.; Ye, Z.; Jimenez, R.; Wang, L.; Wu, H.; Huang, H. DUB3 promotes BET inhibitor resistance and cancer progression by deubiquitinating BRD4. Mol. Cell 2018, 71, 592–605.e594. [Google Scholar] [CrossRef] [PubMed]


| Class of Histone Modifying Enzyme | UPS-Regulated Histone Modifying Enzyme | Target of the Histone Modifying Enzyme | Kinases Involved in UPS-Regulation of Histone-Modifying Enzyme | E3 ligases Regulating Histone Modifying Enzyme | DUBs Regulating Histone Modifying Enzyme | Physiological and Pathophysiological Functions of UPS-Regulation of Histone Modifying Enzyme |
|---|---|---|---|---|---|---|
| Acetylation Enzyme | p300 | H2AK5, H2B, H3, H4 | Mdm2, FBX3, BRMS1 | Cell growth, proliferation, development, differentiation, cell-cycle regulation, DNA damage response, tumorigenesis and apoptosis | ||
| PCAF | Free H3, H3K14, H4K8 | Mdm2 | proliferation, differentiation, apoptosis and cell-cycle progression | |||
| HBO1 | H3K14, H4 | ATM/ATR | CRL4 | cell-cycle progression, DNA replication and proliferation | ||
| Mek1 | FBXW15 | |||||
| Tip60 | H2AK5, H4K16 | UHRF1, EDD1, Mdm2 | USP7 | Cellular signaling, DNA damage repair and transcription | ||
| Deacetylation Enzymes | HDAC1 | H2A, H2B, H3, H4 | Mdm2, Chfr, REN | Development, gene repression, cell cycle, DNA repair, etc | ||
| HDAC2 | H2A, H2B, H3, H4K16 | RLIM, Mule | USP4, USP17 | Differentiation and Development, gene repression, cell cycle, DNA repair, etc | ||
| SETD2 | H3K36 | SPOP | Transcription elongation, RNA processing, DNA repair and damage response, polycomb silencing | |||
| Lysine Methylation Enzymes | SETD3 | H3K36 | GSK3β | FBXW7β | Cell proliferation and tumorigenesis | |
| Set8 | H3K20 | Cdt2 | Cell cycle progression, transcription regulation, DNA repair, genome stability and tumor metastasis | |||
| CDK1 | Cdh1 | |||||
| Skp2 | ||||||
| CK1 | β-TRCP | |||||
| EZH2 | H3K27 | Smurf2, CHIP | USP21, ZRANB1 | Cell proliferation, tumorigenesis, metastasis and stem cell renewal and maintenance | ||
| Jak2 | β-TRCP | |||||
| CDK5 | FBXW7 | |||||
| p38 | Praja1 | |||||
| Lysine Demethylation Enzymes | JMJD2A | H3K9, H3K36 | FBXL4, FBXW2, FBXO22, RNF8 | Replication timing and gemomic stability, DNA damage response, cellular differentiation, and animal development | ||
| LSD1 | H3K4 | Jade2 | USP28, USP22, USP7 | Differentiation, self-renewal and tumor metastasis | ||
| Arginine Methylation Enzymes | PRMT1 | H4R3 | FBXL17, TRIM48, E4B, CHIP | Cell proliferation, progenitor maintenance and tumor metastasis | ||
| PRMT4 | H3R17, H3R26 | Skp2 | Transcription pre- mRNA splicing and cell cycle progression | |||
| Reader | BRD4 | SPOP, CRBN | DUB3 | Cell-cycle, apoptosis, cell proliferation, DNA damage response, autophagy, memory formation and migration and invision |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Qiu, Z.; Wu, Y. Ubiquitin Regulation: The Histone Modifying Enzyme′s Story. Cells 2018, 7, 118. https://0-doi-org.brum.beds.ac.uk/10.3390/cells7090118
Wang J, Qiu Z, Wu Y. Ubiquitin Regulation: The Histone Modifying Enzyme′s Story. Cells. 2018; 7(9):118. https://0-doi-org.brum.beds.ac.uk/10.3390/cells7090118
Chicago/Turabian StyleWang, Jianlin, Zhaoping Qiu, and Yadi Wu. 2018. "Ubiquitin Regulation: The Histone Modifying Enzyme′s Story" Cells 7, no. 9: 118. https://0-doi-org.brum.beds.ac.uk/10.3390/cells7090118