Desulphurisation of Biogas: A Systematic Qualitative and Economic-Based Quantitative Review of Alternative Strategies
Abstract
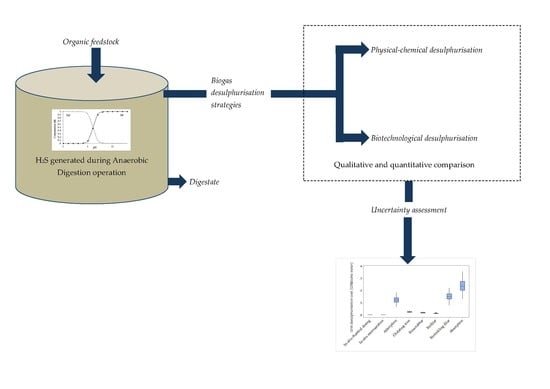
:1. Introduction: Hydrogen Sulphide (H2S) Formation During Anaerobic Digestion and Its Effect on Biogas Utilisation
2. Methodology Employed
2.1. Annual Operating and Annualised Capital Cost per Unit Volume Cost
2.2. A Consideration of the Inherent Uncertainties in the Costing Data
3. Qualitative Review of the Strategies for Biogas Desulphurisation
3.1. Physical–Chemical Desulphurisation Methods
3.1.1. In-Situ Chemical Precipitation
3.1.2. Absorption Technologies
3.1.3. Adsorption Technologies
3.2. Biotechnological Desulphurisation Strategies
3.2.1. In-Situ Microaeration Desulphurisation
3.2.2. Biofiltration Technologies
3.2.3. Phototrophic Sulphur Oxidation
4. Quantitative Analysis of the Desulphurisation Alternatives
Noteworthy Considerations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Okoro, O.V.; Sun, Z.; Birch, J. Catalyst-Free Biodiesel Production Methods: A Comparative Technical and Environmental Evaluation. Sustainability 2018, 10, 127. [Google Scholar] [CrossRef]
- Okoro, O.V.; Sun, Z.; Birch, J. Techno-Economic Assessment of a Scaled-Up Meat Waste Biorefinery System: A Simulation Study. Materials 2019, 12, 1030. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, V.K.; Lo, S.-L. Energy and Resource Recovery from Sludge: Full-Scale Experiences. In Environmental Materials and Waste: Resource Recovery and Pollution Prevention; Academic Press: New York, NY, USA, 2016; pp. 221–244. [Google Scholar]
- Farida, H.; Chang, Y.L.; Hirotsugu, K.; Abdul, A.H.; Yoichi, A.; Takeshi, Y.; Hiroyuki, D. Treatment of Sewage Sludge Using Anaerobic Digestion in Malaysia: Current State and Challenges. Front. Energy Res. 2019, 7, 19. [Google Scholar] [CrossRef]
- Okoro, O.V.; Sun, Z.; Birch, J. Prognostic assessment of the viability of hydrothermal liquefaction as a post-resource recovery step after enhanced biomethane generation using co-digestion technologies. Appl. Sci. 2018, 8, 2290. [Google Scholar] [CrossRef]
- Dinel, H.; Mathur, S.P.; Brown, A.; Lέvesque, M. A Field Study of the Effect of Depth on Methane Production in Peatland Waters: Equipment and Preliminary Results. J. Ecol. 1988, 76, 1083–1091. [Google Scholar] [CrossRef]
- Stams, A.J.M.; Elferink, S.J.W.H.O.; Westermann, P. Metabolic interactions between methanogenic consortia and anaerobic respiring bacteria. Adv. Biochem. Eng. Biotechnol. 2003, 81, 31–56. [Google Scholar] [PubMed]
- Dai, Q.J.; Yi, L.I.; Fang, X.J. Review of desulfurization process for biogas. IOP Conf. Ser. Earth Environ. Sci. 2017, 100, 1–7. [Google Scholar]
- Mackie, R.I.; Stroot, P.G.; Varel, V.H. Biochemical identification and biological origin of key odor components in livestock waste. J. Anim. Sci. 1998, 76, 1331–1342. [Google Scholar] [CrossRef]
- Scheerer, U.; Haensch, R.; Mendel, R.R.; Kopriva, S.; Rennenberg, H.; Herschbach, C. Sulphur flux through the sulphate assimilation pathway is differently controlled by adenosine 5′-phosphosulphate reductase under stress and in transgenic poplar plants overexpressing γ-ECS, SO, or APR. J. Exp. Biol. 2009, 61, 609–622. [Google Scholar] [CrossRef]
- Haghighatafshar, S. Management of H2S in Ananerobic Digestion of Enzyme Pretreated Macro Algae; Lund University: Lund, Sweden, 2012. [Google Scholar]
- Fardeau, M.L.; Ollivier, B.; Patel, B.K.C.; Dwivedi, P.; Ragot, M.; Garcia, J.L. Isolation and characterization of a thermophilic sulfate-reducing bacterium, Desulfotomaculum thermosapovorans sp. nov. Int. J. Syst. Evol. Microbiol. 1995, 45, 218–221. [Google Scholar] [CrossRef]
- Goorissen, H.P.; Boschker, H.T.S.; Stams, A.J.M.; Hansen, T.A. Isolation of thermophilic Desulfotomaculum strains with methanol and sulfite from solfataric mud pools, and characterization of Desulfotomaculum solfataricum sp. nov. Int. J. Syst. Evol. Microbiol 2003, 53, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Stefanie, J.O.E.; Visser, A.; Pol, L.W.H.; Stams, A.J.M. Sulfate Reduction in Methanogenic Bioreactors. FEMS Microbiol. Rev. 1994, 15, 119–136. [Google Scholar]
- Khoshnevisan, B.; Tsapekos, P.; Alfaro, N.; Díaz, I.; Fdz-Polanco, M.; Rafiee, S.; Angelidaki, I. A review on prospects and challenges of biological H2S removal from biogas with focus on biotrickling filtration and microaerobic desulfurization. Biofuel Res. J. 2017, 16, 741–750. [Google Scholar] [CrossRef]
- Sela-Adler, M.; Ronen, Z.; Herut, B.; Antler, G.; Vigderovich, H.; Eckert, W.; Sivan, O. Co-existence of Methanogenesis and Sulfate Reduction with Common Substrates in Sulfate-Rich Estuarine Sediments. Front. Microbiol. 2017, 8, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enning, D.; Garrelfs, J. Corrosion of Iron by Sulfate-Reducing Bacteria: New Views of an Old Problem. Appl. Environ. Microbiol. 2014, 80, 1226–11236. [Google Scholar] [CrossRef] [PubMed]
- Fauque, G.; Ollivier, B. Anaerobes: The sulphate-reducing bacteria as an example of metabolic diversity. In Microbial Diversity and Bioprospecting; ASm Press: Washington, DC, USA, 2004. [Google Scholar]
- Hills, A.G. pH and the Henderson-Hasselbalch equation. Am. J. Med. 1973, 55, 131–133. [Google Scholar] [CrossRef]
- Verma, N.K.; Khanna, S.K.; Kapila, B. Comprehensive Chemistry; Laxmi: New Delhi, India, 2010. [Google Scholar]
- WHO. Hydrogen sulfide. In Air Quality Guidelines for Europe Second Edition; World Health Organisation: Copenhagen, Denmark, 2010; pp. 146–148. [Google Scholar]
- Chaiprapat, S.; Charnnok, B.; Kantachote, D.; Sung, S. Bio-desulfurization of biogas using acidic biotrickling filter with dissolved oxygen in step feed recirculation. Bioresour. Technol. 2015, 179, 429–435. [Google Scholar] [CrossRef]
- Horikawa, M.S.; Rossi, F.; Gimenes, M.L.; Costa, C.M.M.; Silva, M.G.C. chemical absorption of H2S for biogas purification. Braz. J. Chem. Eng. 2004, 21, 415–422. [Google Scholar] [CrossRef]
- Bai, Y.; Bai, Q. Subsea Corrosion and Scale. In Subsea Engineering Handbook, 2nd ed.; Gulf Professional Publishing: Oxford, UK, 2019; pp. 455–487. [Google Scholar]
- CEN. Standard in Development: BS EN 16723-2 Natural Gas and Biomethane for Use in Transport and Biomethane for Injection in the Natural Gas Network Part 2: Automotive Fuel Specifications; European Commitee for Standardisation: Stockholm, Sweden, 2017. [Google Scholar]
- Denyer, D.; Tranfield, D. The Sage Handbook of Organizational Research Methods; Sage: London, UK, 2009; pp. 671–689. [Google Scholar]
- Müller-Langer, F.; Majer, S.; O’Keeffe, S. Benchmarking biofuels—A comparison of technical, economic and environmental indicators. Energy Sustain. Soc. 2014, 4, 1–14. [Google Scholar] [CrossRef]
- Okoro, V.O. Scaled-Up Biodiesel Production from Meat Processing Dissolved Air Flotation Sludge: A Simulation Study. AgriEngineering 2019, 1, 17–43. [Google Scholar] [CrossRef]
- Jenkins, S. Chemical Engineering Plant Cost Index: 2018 Annual Value. Available online: https://www.chemengonline.com/2019-cepci-updates-january-prelim-and-december-2018-final/ (accessed on 29 May 2019).
- Turton, R.C.B.; Whiting, W.B.; Shaeiwitz, J.A. Analysis Synthesis and Design of Chemical Processes; Prentice Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Michailos, S.; McCord, S.; Sick, V.; Stokes, G.; Styring, P. Dimethyl ether synthesis via captured CO2 hydrogenation within the power to liquids concept: A techno-economic assessment. Energy Convers. Manag. 2019, 184, 262–276. [Google Scholar] [CrossRef]
- Díaz, I.; Ramos, I.; Fdz-Polanco, M. Economic analysis of microaerobic removal of H2S from biogas in full-scale sludge digesters. Bioresour. Technol. 2015, 192, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Deshusses, M.A.; Webster, T.S. Construction and Economics of a Pilot/Full-Scale Biological Trickling Filter Reactor for the Removal of Volatile Organic Compounds from Polluted Air. J. Air Waste Manag. Assoc. 2011, 50, 1947–1956. [Google Scholar] [CrossRef]
- Nägele, H.J.; Steinbrenner, J.; Hermanns, G.; Holstein, V.; Haag, N.L.; Oechsner, H. Innovative additives for chemical desulphurisation in biogas processes: A comparative study on iron compound products. Biochem. Eng. J. 2017, 121, 181–187. [Google Scholar] [CrossRef]
- Erdirencelebi, D.; Kucukhemek, M. Control of H2S in full-scale anaerobic digesters using iron(III) chloride: Performance, origin and effects. Water SA 2018, 44, 176–183. [Google Scholar] [CrossRef]
- Schäfer, F.; Dittrich-Zechendorf, M.; Leiker, M.; Pröter, J. Powdery ferric compounds for biogas process desulphurisation. Landtechnik 2017, 72, 39–48. [Google Scholar]
- KRONOS. Hydrogen Sulfide Elimination from Biogas; KRONOS International Inc.: Dallas, TX, USA, 2014. [Google Scholar]
- Jiang, H.; Li, T.; Stinner, W.; Nie, H. Selection of in-situ Desulfurizers for Chicken Manure Biogas and Prediction of Dosage. Pol. J. Environ. Stud. 2017, 26, 155–161. [Google Scholar] [CrossRef]
- SevernWye. Introduction to Production of Biomethane from Biogas: A Guide for England and Wales; SevernWye Agency: Bangor, Wales, 2017. [Google Scholar]
- Lupitskyy, R.; Alvarez-Fonseca, D.; Herde, Z.D.; Satyavolu, J. In-situ prevention of hydrogen sulfide formation during anaerobic digestion using zinc oxide nanowires. J. Environ. Chem. Eng. 2018, 6, 110–118. [Google Scholar] [CrossRef]
- Allegue, L.B.; Hinge, J.H. Biogas Upgrading Evaluation for H2S Removal; Danish Technology Institute: Taastrup, Denmark, 2014. [Google Scholar]
- Krayzelova, L.; Bartacek, J.; Díaz, I.; Jeison, D.; Volcke, E.I.P.; Jenicek, P. Microaeration for hydrogen sulfide removal during anaerobic treatment: A review. Rev. Environ. Sci. Biotechnol. 2015, 14, 703–725. [Google Scholar] [CrossRef]
- NYSERDA. Assessment of Biochemical Process Controls for Reduction of Hydrogen Sulfide Concentrations in Biogas from Farm Digesters; New York State Energy Research and Development Authority: New York, NY, USA, 2012. [Google Scholar]
- Smith, J.A.; Carliell-Marquet, C.M. The digestibility of iron-dosed activated sludge. Bioresour. Technol. 2008, 99, 8585–8592. [Google Scholar] [CrossRef]
- Al-Imarah, K.A.; Lafta, T.M.; Jabr, A.K.; Mohammad, A.N. Desulfurization for Biogas Generated by Lab Anaerobic Digestion unit. J. Agric. Vet. Sci. 2017, 10, 2319–2372. [Google Scholar]
- Ofverstrom, S.; Dauknys, R.; Sapkaitė, I. The effect of iron salt on anaerobic digestion and phosphate release to sludge liquor. Environ. Prot. Eng. 2011, 3, 123–126. [Google Scholar]
- Khanal, S.K.; Huang, J. Online Oxygen Control for Sulfide Oxidation in Anaerobic Treatment of High-Sulfate Wastewater. Water Environ. Res. 2006, 78, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A Review of Biogas Utilisation, Purification and Upgrading Technologies. Waste Biomass Valoris. 2017, 8, 267–283. [Google Scholar] [CrossRef] [Green Version]
- Lein, C.; Wang, M.; Lin, W. Study on the removal of H2S from biogas biomaterial using water scrubbing. Trans. Tech. Publ. 2017, 723, 599–603. [Google Scholar]
- ToolBox, E. Solubility of Gases in Water. 2008. Available online: https://www.engineeringtoolbox.com/gases-solubility-water-d_1148.html (accessed on 23 April 2019).
- Nie, H.; Jiang, H.; Chong, D.; Wu, Q.; Xu, C.; Zhou, H. Comparison of Water Scrubbing and Propylene Carbonate Absorption for Biogas Upgrading Process. Energy Fuels 2013, 27, 3239–3245. [Google Scholar] [CrossRef]
- Dyment, J.; Watanasiri, S. Acid Gas Cleaning Using DEPG Physical Solvents: Validation with Experimental and Plant Data; Aspentech: Bedford, UK, 2015. [Google Scholar]
- Petersson, A.; Wellinger, A. Biogas Upgrading Technologies–Developments and Innovations. Task 37-Energy from Biogas and Landfill Gas; EA Bioenergy: Aadorf, Switzerland, 2009. [Google Scholar]
- Deshmukh, G.M.; Shete, A.; Pawar, D.M. Oxidative Absorption of Hydrogen Sulfide using Iron-chelate Based Process: Chelate Degradation. J. Anal. Bioanal. Tech. 2012, 3, 138. [Google Scholar] [CrossRef]
- Frare, L.; Vieira, M.; Silva, M.; Pereira, N.; Gimenes, M. Hydrogen Sulfide Removal from Biogas Using Fe/EDTA Solution: Gas/Liquid Contacting and Sulfur Formation. Environ. Prog. Sustain. Energy 2009, 29, 34–41. [Google Scholar] [CrossRef]
- Neumann, D.W.; Lynn, S. Oxidative absorption of H2S and O2 by iron chelate solutions. Am. Inst. Chem. Eng. 1984, 30, 62–69. [Google Scholar] [CrossRef]
- Maia, D.C.S.; Niklevicz, R.R.; Arioli, R.; Frare, L.M.; Arroyo, P.A.; Gimenes, M.L.; Pereira, N.C. Removal of H2S and CO2 from biogas in bench scale and the pilot scale using a regenerable Fe-EDTA solution. Renew. Energy 2017, 109, 188–194. [Google Scholar] [CrossRef]
- Krischan, J.; Makaruk, A.; Harasek, M. Design and scale-up of an oxidative scrubbing process for the selective removal of hydrogen sulfide from biogas. J. Hazard. Mater. 2012, 215, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Papurello, D. Biogas Cleaning: Activated Carbon Regeneration for H2S Removal. Clean Technol. 2018, 1, 40–57. [Google Scholar] [CrossRef]
- Magomnang, A.S.M.; Villanueva, E.P. Removal of Hydrogen Sulfide from Biogas using Dry Desulfurization Systems. In Proceedings of the International Conference on Agricultural, Environmental and Biological Sciences, Phuket, Thailand, 24–25 April 2014. [Google Scholar]
- UNIDO. Biogas to Biomethane. 2017. Available online: https://www.biogas-to-biomethane.com/Download/BTB.pdf (accessed on 30 April 2019).
- Cherif, H. Study and Modeling of Separation Methods H2S from Methane, Selection of a Method Favoring H2S Valorization; PSL Research University: Paris, France, 2016. [Google Scholar]
- Rocca, M.A. Surface Science and Nanostructuring/Adsorption at Surfaces. 2012. Available online: http://www.fisica.unige.it/~rocca/Didattica/Surface%20Science%20and%20Nanostructuring/6%20adsorption%20at%20surfaces.pdf (accessed on 30 April 2019).
- Al Mamun, M.R.; Torii, S. Removal of Hydrogen Sulfide (H2S) from Biogas Using Zero-Valent Iron. J. Clean Energy Technol. 2015, 3, 428–432. [Google Scholar] [CrossRef]
- Sitthikhankaew, R.; Predapitakkun, S.; Kiattikomol, R.; Pumhiran, S.; Assabumrungrat, S.; Laosiripojana, N. Comparative Study of Hydrogen Sulfide Adsorption by using Alkaline Impregnated Activated Carbons for Hot Fuel Gas Purification. Energy Procedia 2011, 9, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Louhichi, S.; Ghorbel, A.; Takfaoui, A.; Chekir, H.; Trabelsi, N.; Khemakhem, S. Alkaline activated carbon as adsorbents of hydrogen sulfide gases from chimney of phosphoric units. J. Mater. Environ. Sci. 2018, 9, 2686–2691. [Google Scholar]
- Kwaśny, J.; Balcerzak, W.; Rezka, P. Application of zeolites for the adsorptive biogas desulfurisation. Tech. Trans. Environ. Eng. 2015, 39–45. [Google Scholar] [CrossRef]
- Micoli, L.; Bagnasco, G.; Turco, M. H2S removal from biogas for fuelling MCFCs: New adsorbing materials. Int. J. Hydrog. Energy 2014, 39, 1783–1787. [Google Scholar] [CrossRef]
- Feldbauer, S.L. Steam Treating; Enhancing the Surface Properties of Metal Components; Aabbott Furnace Company: St. Marys, PA, USA, 2003. [Google Scholar]
- Wang, D. Breakthrough Behavior of H2S Removal with an Iron Oxide Based CG-4 Adsorbent in a Fixed-Bed Reactor; University of Saskatchewan: Saskatoon, SK, Canada, 2008. [Google Scholar]
- Raabe, T.; Erler, R.; Kureti, S.; Krause, H. Oxygen Removal during Biogas Upgrading using iron-based Adsorbents. In Proceedings of the International Gas Union Research Conference, Copenhagen, Denmark, 17–19 September 2014. [Google Scholar]
- Pourzolfaghar, H.; Ismail, M.; Izhar, S.; MagharehEsfahan, Z. Review of H2S Sorbents at Low-Temperature Desulfurization of Biogas. Int. J. Chem. Environ. Eng. 2004, 5, 22–28. [Google Scholar]
- TVT. Biogas to Biomethane Technology Review; Vienna University of Technology: Vienna, Austria, 2012. [Google Scholar]
- Huertas, J.I.; Giraldo, N.; Izquierdo, S. Removal of H2S and CO2 from Biogas by Amine Absorption, Mass Transfer in Chemical Engineering Processes; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Paolini, V.; Petracchini, F.; Guerriero, E.; Bencini, A.; Drigo, S. Biogas cleaning and upgrading with natural zeolites from tuffs. Environ. Technol. 2016, 37, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Thanapong, D. Micro-Aeration for Hydrogen Sulfide Removal from Biogas; Iowa State University: Iowa Ames, IA, USA, 2009. [Google Scholar]
- Tang, Y.; Shigematsu, T.; Morimura, S.I.; Kida, K. The effects of micro-aeration on the phylogenetic diversity of microorganisms in a thermophilic anaerobic municipal solid-waste digester. Water Res. 2004, 38, 2537–2550. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiao, W. Transformations and impacts of ammonia and H2S in anaerobic digestion reactors. In Ananerobic Biotechnology: Environmental Protection and Resource Recovery; Imperial College Press: London, UK, 2015; pp. 109–133. [Google Scholar]
- Huber, B.; Herzog, B.; Drewes, J.E.; Koch, K.; Müller, E. Characterization of sulfur oxidizing bacteria related to biogenic sulfuric acid corrosion in sludge digesters. BMC Microbiol. 2016, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Kleinjan, W. Biologically Produced Sulphur Particles and Polysulphide Sulphide Ions. Effects on a Biotechnological Process for the Removal of Hydrogen suphide from Gas Streams. Ph.D. Thesis, Wageningen Universiteit, Wageningen, The Netherlands, 2005. [Google Scholar]
- Díaz, I.; Pérez, S.I.; Ferrero, E.M.; Fernández–Polanco, F. Effect of oxygen dosing point and mixing on the microaerobic removal of H2S in sludge digesters. Bioresour. Technol. 2011, 102, 3768–3775. [Google Scholar] [CrossRef] [PubMed]
- Duangmanee, T. Micro-Aeration for Hydrogen Sulphide Removal from Biogas. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2009. [Google Scholar] [CrossRef]
- Guerrero, L.; Montalvo, S.; Huiliñir, C.; Campos, J.L.; Barahona, A.; Borja, R. Advances in the biological removal of sulphides from aqueous phase in anaerobic processes: A review. Environ. Rev. 2015, 24, 84–100. [Google Scholar] [CrossRef]
- Dıaz, I.; Lopes, A.C.; Perez, S.I.; Fdz-Polanco, M. Determination of the optimal rate for the microaerobic treatment ofseveral H2S concentrations in biogas from sludge digesters. Water Sci. Technol. 2011, 64, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Van den Ende, F.P.; van Gemerden, H. Sulfide oxidation under oxygen limitation by a Thiobacillus thioparus isolated from a marine microbial mat. FEMS Microbiol. Ecol. 1993, 13, 69–77. [Google Scholar]
- Jeníček, P.; Horejš, J.; Pokorná-Krayzelová, L.; Bindzar, J.; Bartáček, J. Simple biogas desulfurization by microaeration—Full scale experience. Anaerobe 2017, 46, 41–45. [Google Scholar] [CrossRef]
- Zitomer, D.H.; Shrout, J.D. High-sulfate, high chemical oxygen demand wastewater treatment using aerated methanogenic fluidized beds. Water Environ. Res. 2000, 72, 90–97. [Google Scholar] [CrossRef]
- Jenicek, P.; Koubova, J.; Bindzar, J.; Zabranska, J. Advantages of anaerobic digestion of sludge in microaerobic conditions. Water Sci. Technol. 2010, 62, 427–434. [Google Scholar] [CrossRef]
- Karhadkar, P.P.; Audic, J.; Faup, G.M.; Khanna, P. Sulfide and sulfate inhibition of methanogenesis. Water Res. 1987, 21, 1061–1066. [Google Scholar] [CrossRef]
- Parker, M.L. Method for Removing Hydrogen Sulfide from Sour Gas and Converting It to Hydrogen and Sulfuric Acid. Ph.D. Thesis, Stanford University, Stanford, CA, USA, 1 June 2010. [Google Scholar]
- Valdés, F.; Camiloti, P.R.; Rodriguez, R.P.; Delforno, T.P.; Carrillo-Reyes, J.; Zaiat, M.; Jeison, D. Sulfide-oxidizing bacteria establishment in an innovative microaerobic reactor with an internal silicone membrane for sulfur recovery from wastewater. Biodegradation 2016, 27, 119–130. [Google Scholar] [CrossRef]
- Pokorna-Krayzelova, L.; Bartacek, J.; Theuri, S.N.; Gonzalez, C.A.S.; Prochazk, J.; Volcke, E.I.P.; Jenicek, P. Microaeration through a biomembrane for biogas desulfurization: Lab-scale and pilot-scale experiences. Environ. Sci. Water Res. Technol. 2018, 4, 1190–1200. [Google Scholar] [CrossRef]
- Ramos, I.; Pérez, R.; Fdz-Polanco, M. Microaerobic desulphurisation unit: a new biological system for the removal of H2S from biogas. Bioresour. Technol. 2013, 142, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Lópeza, L.R.; Dorado, A.D.; Mora, M.; Gamisans, X.; Lafuente, J.; Gabriel, D. Modeling an aerobic biotrickling filter for biogas desulfurization through a multi-step oxidation mechanism. Chem. Eng. J. 2016, 294, 447–457. [Google Scholar] [CrossRef] [Green Version]
- Montebello, A.M.; Mora, M.; López, L.R.; Bezerra, T.; Gamisans, X.; Lafuente, J.; Baeza, M.; Gabriel, D. Aerobic desulfurization of biogas by acidic biotrickling filtration in a randomly packed reactor. J. Hazard. Mater. 2014, 280, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Barbusiński, K.; Kalemba, K. Use of biological methods for removal of H2S from biogas in wastewater treatment plants—A review. Arch. Civ. Eng. Environ. 2016, 9, 103–112. [Google Scholar] [CrossRef]
- Soreanu, G.; Béland, M.; Falletta, P.; Ventresca, B.; Seto, P. Evaluation of different packing media for anoxic H2S control in biogas. Environ. Technol. 2009, 30, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, D.; Cox, H.H.J.; Deshusses, M.A. Conversion of Full-Scale Wet Scrubbers to Biotrickling Filters for Control at Publicly Owned Treatment Works. J. Environ. Eng. 2004, 130, 1110–1117. [Google Scholar] [CrossRef]
- Qiu, X.; Deshusses, M.A. Performance of a monolith biotrickling filter treating high concentrations of H2S from mimic biogas and elemental sulfur plugging control using pigging. Chemosphere 2017, 186, 790–797. [Google Scholar] [CrossRef]
- Tayar, S.P.; Guerrero, R.B.C.; Hidalgo, L.F.; Bevilaqua, D. Evaluation of Biogas Biodesulfurization Using Different Packing Materials. Chemengineering 2019, 3, 27. [Google Scholar] [CrossRef]
- Devinny, J.S.; Deshusses, M.A.; Webster, T.S. Biofiltration for Air Pollution Control; CRC-Lewis Publishers: Boca Raton, FL, USA, 1999. [Google Scholar]
- Liu, D.H.F. Environmental Engineers’ Handbook; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Webster, T.S.; Devinny, J.S. Biofiltration of Odors, Toxics and Volatile Organic Compounds from Publicly Owned Treatment Works. Environ. Prog. 1996, 15, 141–147. [Google Scholar] [CrossRef]
- Converse, B.M.; Schroeder, E.D.; Iranpour, R.; Cox, H.H.J.; Deshusses, M.A. Odor and Volatile Organic Compound Removal from Wastewater Treatment Plant Headworks Ventilation Air Using a Biofilter. Water Environ. Res. 2003, 75, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Dumont, E. H2S removal from biogas using bioreactors: A review. Int. J. Energy Environ. 2015, 6, 479–498. [Google Scholar]
- Fernández, M.; Ramírez, M.; Gómez, J.M.; Cantero, D. Biogas biodesulfurization in an anoxic biotrickling filter packed with open-pore polyurethane foam. J. Hazard. Mater. 2014, 264, 529–535. [Google Scholar] [CrossRef]
- Almenglo, F.; Bezerra, T.; Lafuente, J.; Gabriel, D.; Ramírez, M.; Cantero, D. Effect of gas-liquid flow pattern and microbial diversity analysis of a pilot-scale biotrickling filter for anoxic biogas desulfurization. Chemosphere 2016, 157, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.M.; Hargreaves, R.; Witherspoon, J.; Ong, H.; Burrowes, P.; Easter, C.; Dickey, J.; James, F.; MacPherson, L.; Porter, R.; et al. Identifying and Controlling Municipal Wastewater Odor Phase I: Literature Search and Review; IWA publishing: Alexandria, Egypt, 2003. [Google Scholar]
- Schiavon, M.; Ragazzi, M.; Torretta, V.; Rada, E.C. Comparison between conventional biofilters and biotrickling filters applied to waste bio-drying in terms of atmospheric dispersion and air quality. Environ. Technol. 2015, 37, 975–982. [Google Scholar] [CrossRef]
- Pathak, N.; Mahajan, P.V. Ethylene Removal from Fresh Produce Storage: Current Methods and Emerging Technologies. Ref. Modul. Food Sci. 2017. [Google Scholar] [CrossRef]
- Oyarzún, P.; Arancibia, F.; Canales, C.; Aroca, G.E. Biofiltration of high concentration of H2S using Thiobacillus thioparus. Process Biochem. 2003, 39, 165–170. [Google Scholar] [CrossRef]
- SU, J.J.; Chen, Y.J.; Chang, Y.C. A study of pilot scale biogas bio-filter system for utilisation on pigs farms. J. Agric. Sci. 2014, 152, 217–224. [Google Scholar] [CrossRef]
- Groenestijn, J.W.V. Biotechniques for Air Pollution Control: Past, Present and Future Trend; University of La Coruña Publisher: La Coruña, Spain, 2005. [Google Scholar]
- Koe, L.C.C. Evaluation of a pilot-sclea bioscrubber for the removal of H2S. Water Environ. J. 2000, 14, 432–435. [Google Scholar] [CrossRef]
- Koutinas, M.; Peeva, L.G.; Livingston, A.G. An attempt to compare the performance of bioscrubbers and biotrickling filters for degradation of ethyl acetate in gas streams. J. Chem. Technol. Biotechnol. 2005, 80, 1252–1260. [Google Scholar] [CrossRef]
- Van-Groenestijn, J.W. Bioscrubbers. In Bioreactors for Waste Gas Treatment. Environmental Pollution; Springer: Dordrecht, The Netherlands, 2001; pp. 133–162. [Google Scholar]
- LeCloirec, P.; Humeau, P. Bioscrubbers. In Air Pollution Prevention and Control: Bioreactors and Bioenergy; John Wiley & Sons: West Sussex, UK, 2013; pp. 139–153. [Google Scholar]
- Behera, B.C.; Mishra, R.R.; Dutta, S.K.; Thatoi, H.N. Sulphur oxidising bacteria in mangrove ecosystem: A review. Afr. J. Biotechnol. 2014, 13, 2897–2907. [Google Scholar] [Green Version]
- Tang, K.; Baskaran, V.; Nemati, M. Bacteria of the sulphur cycle: An overview of microbiology, biokinetics and their role in petroleum and mining industries. Biochem. Eng. J. 2009, 44, 73–94. [Google Scholar] [CrossRef]
- Simon, J.; Kroneck, P.M.H. Chapter Two—Microbial Sulfite Respiration. In Advances in Microbial Physiology; Academic Press: Oxford, UK, 2013; pp. 45–117. [Google Scholar]
- Lampe, D.G.; Zhang, T.C. Evaluation of sulfur-based autotrophic denitrification. In Proceedings of the Great Plains/Rocky Mountain Hazardous Substance Research Center (HSRC)/Waste-management Education & Research Consortium (WERC) JointConference on the Environment, Albuquerque, NM, USA, 21–23 May 1996. [Google Scholar]
- Hayakawa, A.; Hatakeyama, M.; Asano, R.; Ishikawa, Y.; Hidaka, S. Nitrate reduction coupled with pyrite oxidation in the surface sediments of a sulfide-rich ecosystem. JGR Biogeosci. 2013, 118, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Dolejs, P.; Paclík, L.; Maca, J.; Pokorna, D.; Zabranska, J.; Bartacek, J. Effect of S/N ratio on sulfide removal by autotrophic denitrification. Appl. Microbiol. Biotechnol. 2015, 99, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Soreanu, G.; Béland, M.; Falletta, P.; Ventresca, B.; Seto, P. Laboratory pilot scale study for H2S removal from biogas in an anoxic biotrickling filter. Water Sci. 2008, 57, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Khanongnuch, R.; Di Capua, F.; Lakaniemi, A.; Rene, E.R.; Lensa, P.N.L. H2S removal and microbial community composition in an anoxic biotrickling filter under autotrophic and mixotrophic conditions. J. Hazard. Mater. 2019, 367, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; Malhautier, L.; Fanlo, J.; Quijano, G. Biological technologies for the treatment of atmospheric pollutants. Int. J. Environ. Anal. Chem. 2015, 95, 950–967. [Google Scholar] [CrossRef]
- López, L.R.; Dorado, A.D.; Mora, M.; Prades, L.I.; Gamisans, X.; Lafuente, J.; Gabriel, D. Modelling biotrickling filters to minimize elemental sulfur accumulation during biogas desulfurization under aerobic conditions. In Proceedings of the 7th European Meeting on Chemical Industry and Environment, Tarragona, Spain, 10–12 June 2015. [Google Scholar]
- Mezzari, M.P.; Da Silva, M.L.B. Sulfide Removal from Biogas by Sulphur Oxidising Bacteria; Internacional Sobre Gerenciamento Deresíduos Agropecuários e Agroindustriais: SÃO Pedro, São Paulo, Brazil, 2013. [Google Scholar]
- Syed, M.; Soreanu, G.; Falletta, P.; Béland, M. Removal of hydrogen sulfide from gas streams using biological processes—A review. Can. Biosyst. Eng. 2006, 48, 2. [Google Scholar]
- Cork, D.; Mather, J.; Maka, A.; Srnak, A. Control of oxidative sulfur metabolism of Chlorobium limola forma thiosulfatophilum. Appl. Environ. Microbiol. 1985, 49, 269–272. [Google Scholar]
- Pokorna, D.; Zabranska, J. Sulfur-oxidizing bacteria in environmental technology. Biotechnol. Adv. 2015, 33, 1246–1259. [Google Scholar] [CrossRef]
- Massé, A.; Pringault, O.; Wit, R. Experimental Study of Interactions between Purple and Green Sulfur Bacteria in Sandy Sediments Exposed to Illumination Deprived of Near-Infrared Wavelengths. Appl. Environ. Microbiol. 2002, 68, 2972–2981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerardi, M.H.; Lytle, B. Purple and Green Sulfur Bacteria. In The Biology and Troubleshooting of Facultative Lagoons; John Wiley & Sons: New York, NY, USA, 2015; pp. 73–76. [Google Scholar]
- Burns, A.S.; Padilla, C.C.; Pratte, Z.A.; Gilde, K.; Regensburger, M.; Hall, E.; Dove, A.D.M.; Stewart, F.J. Broad Phylogenetic Diversity Associated with Nitrogen Loss through Sulfur Oxidation in a Large Public Marine Aquarium. Appl. Environ. Microbiol. 2018, 84, e01250-18. [Google Scholar] [CrossRef] [PubMed]
- Pfenning, N. Phototropic green and purple bacteria: A comparative, systematic survey. Ann. Rev. Microbiol. 1977, 31, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G.; Ferguson, S.J. Photosynthetic Generators of Protonmotive Force. In Bioenergetics, 4th ed.; Academic Press: Oxford, UK, 2013; pp. 159–196. [Google Scholar]
- Müller, J.; Overmann, J. Close interspecies interactions between prokaryotes from sulfureous environments. Front. Microbiol. 2011, 2, 146. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.A.; Kuenen, J.G. Denitrification by obligate and facultative autotrophs. In Autotrophic Microbiology and One-Carbon Metabolism Vol. 1; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2012; pp. 93–117. [Google Scholar]
- Rawat, R.; Rawat, S. Colorless sulfur oxidizing bacteria from diverse habitats. Adv. Appl. Sci. Res. 2015, 6, 230–235. [Google Scholar]
- Canfield, D.E.; Kristensen, E.; Thamdrup, B. The Sulfur Cycle. In Advances in Marine Biology; Academic Press: Cambridge, MA, USA, 2005; pp. 313–381. [Google Scholar]
- Barak, Y.; Tal, Y.; Rijn, J. Light-Mediated Nitrite Accumulation during Denitrification by Pseudomonas sp. Strain JR12. Appl. Environ. Microbiol. 1998, 64, 813–817. [Google Scholar]
- Qambrani, N.A.; Oh, S. Effect of Dissolved Oxygen Tension and Agitation Rates on Sulfur-Utilizing Autotrophic Denitrification: Batch Tests. Appl. Biochem. Biotechnol. 2013, 169, 181–191. [Google Scholar] [CrossRef]
- Kuenen, J.G.; Robertson, L.A.; Gemerden, H. Microbial Interactions among Aerobic and Anaerobic Sulfur-Oxidizing Bacteria. In Advances in Microbial Ecology: Volume 8; Springer: Boston, MA, USA, 1985; pp. 1–54. [Google Scholar]
- Gemerden, H. Production of elemental sulfur by green and purple sulfur bacteria. Arch. Microbiol. 1986, 146, 52–56. [Google Scholar] [CrossRef]
- Holkenbrink, C.; Barbas, S.O.; Mellerup, A.; Otaki, H.; Frigaard, N.U. Sulfur globule oxidation in green sulfur bacteria is dependent on the dissimilatory sulfite reductase system. Microbiology 2011, 157, 1229–1239. [Google Scholar] [CrossRef] [Green Version]
- Fenchel, T.; King, G.M.; Blackburn, T.H. Aquatic Sediments. In Bacterial Biogeochemistry, 3rd ed.; Elsevier: London, UK, 2012; pp. 121–142. [Google Scholar]
- Garrido, M.M. Characterisation of S-Oxidising Biomass through Respirametric Techniques under Anoxic and Aerobic Conditions; Univesitat Autonoma de Barcelona: Barcelona, Spain, 2014. [Google Scholar]
- Pringault, O.; Kuhlt, M.; Wit, R.; Caumettel, P. Growth of green sulphur bacteria in experimental benthic oxygen, sulphide, pH and light gradients. Microbiology 1998, 144, 1051–1061. [Google Scholar] [CrossRef] [Green Version]
- Bharathi, P.A.L. Sulfur Cycle. In Encyclopedia of Ecology; Academic Press: Oxford, UK, 2008; pp. 3424–3431. [Google Scholar]
- Mahmood, Q.; Zheng, P.; Cai, J.; Wu, D.; Hu, B.; Li, J. Anoxic sulfide biooxidation using nitrite as electron acceptor. J. Hazard. Mater. 2007, 147, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Wahlund, T.M.; Tabita, F.R. The reductive tricarboxylic acid cycle of carbon dioxide assimilation: initial studies and purification of ATP-citrate lyase from the green sulfur bacterium Chlorobium tepidum. J. Bacteriol. 1997, 179, 4859–4867. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, L.J. The evolution of photosynthesis.again? Phil. Trans. R. Soc. B 2008, 363, 2787–2801. [Google Scholar] [CrossRef] [PubMed]
- Kuenen, J.G. Colourless sulphur bacteria and their role in the sulphur cycle. Plant Soil 1975, 43, 49–76. [Google Scholar] [CrossRef]
- Okafor, N. Environmental Microbiology of Aquatic and Waste Systems; Springer Science: Asheville, NC, USA, 2011. [Google Scholar]
- Chemengonline. Economic Indicators. Available online: https://www.chemengonline.com/mediakit/wp-content/uploads/2016/01/EconomicIndicatorSampleSept17.pdf (accessed on 29 May 2019).
- Shelford, T.; Gooch, C.; Choudhury, A.; Lansing, S. A Technical Reference Guide for Dairy-Derived Biogas Production, Treatment and Utilization. 2019. Available online: https://enst.umd.edu/sites/enst.umd.edu/files/_docs/FarmerbiogashandbookFinal.pdf (accessed on 20 July 2019).
- Connolly, E.L.; Guerinot, M.L. Iron stress in plants. Genome Biol. 2002, 3. [Google Scholar] [CrossRef]
- Fdz-Polanco, M.; Diaz, I.; Perez, S.I.; Lopes, A.C.; Fdz-Polanco, F. H2S removal in the anaerobic digestion of sludge by micro-aerobic processes: Pilot plant experience. Water Sci. Technol. 2009, 60, 3045–3050. [Google Scholar] [CrossRef] [PubMed]
- Jenicek, P.; Celis, C.A.; Krayzelova, L.; Anferova, N.; Pokorna, D. Improving products of anaerobic sludge digestion by microaeration. Water Sci. Technol. 2014, 69, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, L.D.; Manassa, P.D.M.; Fitzgerald, S.K. Oxidation reduction potential as a parameter to regulate micro-oxygen injection into anaerobic digester for reducing H2S concentration in biogas. Bioresour. Technol. 2014, 173, 443–447. [Google Scholar] [CrossRef]
- Cano, P.I.; Colón, J.; Ramírez, M.; Lafuente, J.; Gabriel, D.; Cantero, D. Life cycle assessment of different physical-chemical and biological technologies for biogas desulfurization in sewage treatment plants. J. Clean. Prod. 2018, 181, 663–674. [Google Scholar] [CrossRef]
- Santos-Clotas, E.; Cabrera-Codony, A.; Castillo, A.; Martín, M.J.; Poch, M.; Monclus, H. Environmental Decision Support System for Biogas Upgrading to Feasible Fuel. Energies 2019, 12, 1546. [Google Scholar] [CrossRef]






| Differentiating Parameter | Physisorption | Chemisorption |
|---|---|---|
| Bonding forces | Van der Waals forces bond the adsorbate and absorbent with the adsorbate molecule retaining its gas phase electronic structure. | Stronger covalent forces are employed leading to perturbation of the molecular electronic structure of the adsorbate molecule |
| Adsorption heat | The enthalpy change during the adsorption process is usually low and ranges from 30 to 40 kJ·mol−1. | The enthalpy change during the adsorption process is usually high and ranges from 80 to 800 kJ·mol−1. |
| Adsorption layers | Multi-layer adsorption typically occurs. | Monolayer adsorption typically occurs. |
| Temperature requirement | Varies with the specific adsorbate and adsorbent employed with low temperatures considered as favourable. | Higher temperatures considered favourable. |
| Kinetics | It is a reversible process. | Irreversible as new compounds are formed at the adsorbent surface. |
| Some Properties | Sulphur Oxidising Bacteria | |||
|---|---|---|---|---|
| Photoautotrophs | Chemolithotrophs a | References | ||
| Green Sulphur Bacteria | Purple Sulphur Bacteria | |||
| Sulphide oxidation pathway | Reduces carbon dioxide to carbohydrates via the H2S oxidation. | Also capable of undergoing the phototropic conversion of carbon dioxide to carbohydrates via H2S oxidation. | May oxidize inorganic sulphur compounds using oxygen, generating energy (aerobic species such as Beggiatoa sp) May also oxidize inorganic sulphur compounds using nitrogen oxides for energy generation. Also called autotropic dentrifiers (i.e., anaerobic species such as Thiomicrospira denitrificans. | [133,134] |
| Physiology | Green bacteria are either non-motile green or gliding filamentous green bacteria. Gas vesicles are present and responsible for enhanced buoyancy. Chlorosome complexes are also present and that serve as ‘photosynthetic antennas’. | Most purple bacteria are flagellated. Typically, gas vesicles and chlorosomes are not present. | The chemolithotrophs are typically considered colourless due to the absence of photopigments. They may be motile, filamentous organisms (i.e., Thiobacillus denitrificans, Beggiatoa) as the mobility assists in migration to regions of higher oxygen concentration. They may also exist as non-motile organisms (i.e., Thiomicrospira denitrificans) | [119,135,136,137,138,139] |
| Light response | Green sulphur bacteria are phototrophs thus require light. They absorb longer wavelengths of light than purple sulphur bacteria since have they have special adaptations to low light. A notable example is the green bacteria is Chlorobium phaeobacteroides which is reported to be capable of surviving under a light level of <0.25 μmol photons m−2 s−1. | Purple sulphur bacteria are phototrophs thus also require light. They require shorter wavelengths of light, highlighting the need for higher energy requirement by the purple bacteria. | Photo-inhibition even in low light intensities has been reported in previous studies, with protection from light considered desirable. | [133,140,141] |
| Oxygen tolerance | Strict obligate anaerobes | Can survive in the presence of molecular oxygen (facultative) | Some are dependent on oxygen, thus they are often localised at the interface between aerobic and anaerobic zones where low concentrations of oxygen and sulphide can coexist (i.e., Beggiatoa sp and Thioploca sp are aerobic). Other colorless bacteria may exist as absolute obligates (i.e., Thiomicrospira denitrificans) with some species (i.e., Thiobacillus denitrificans) capable of consuming low oxygen concentrations. | [132,139,142,143] |
| Sulphur availability | Elemental sulphur is deposited extracellularly, free of any encapsulation by proteins. If the sulphides are depleted in the substrate, extracellular sulphur globules may be oxidised completely to sulphate. | Elemental Sulphur is typically deposited intracellularly as spherical particles and is encapsulated by proteins. The sulphur is further oxidised and released from the cells as sulphates. Some species of the purple sulphur bacteria such as Ectothiorhodospira, Halorhodospira, Thiorhodospira also have the capability to deposit elemental sulphur extracellularly. | There may be an intracellular accumulation of elemental sulphur. In some cases, up to 30% of dry cell mass is sulphur (i.e., Beggiatoa sp). Large internal vacuoles facilitated the storage of sulphur within the cell wall | [131,144,145,146,147] |
| Sulphide tolerance | Green sulphur bacteria exhibit a high tolerance for high concentrations of sulphides in solution of up to 5–10 mM. This is largely because sulphide oxidation occurs extracellularly. | High sulphide concentrations (5–10 mM) are considered toxic to purple sulphur bacteria largely because sulphides are oxidised internally. | They do not tolerate very high sulphide concentrations (i.e.,~>1920 mg/L). | [146,148,149,150] |
| CO2 fixation pathway | CO2 fixation is typically achieved via the reductive tricarboxylic acid (RTCA) cycle. | The reductive pentose phosphate (also called Calvin-Benson- Bassham) cycle is typically employed in CO2 fixation. | CO2 fixation is typically achieved via the reductive ribulosediphosphate (Calvin) cycle. Some may also be able to utilise small amounts of exogenous organic carbon as carbon sources. | [143,151,152,153] |
| Bacteriochlorophyll forms of types a,b,c,d,e and g (letters to illustrate slight changes in structure) | Bacteriochlorophylls of c, d, and e are present | Bacteriochlorophylls of a and b are present | Photopigments are absent. | [154] |
| Desulphurisation Method a | Total Operating Cost (US $/y) b | Equipment Purchase Cost (US $) b |
|---|---|---|
| In-situ chemical dosing | 12,684.672 | 0 d |
| In-situ microaeration | 3850.704 c | 25,184.7 c |
| Desulphurisation Method | Capital Cost per Unit Gas Volume (US $ per m3) a | Operating Cost per Unit Gas Volume (US $ per m3) a |
|---|---|---|
| Chelating iron b | 0.170 | 0.070 |
| Bioscrubbers | 0.160 | 0.020 |
| Biofilter | 0.090 | 0.030 |
| Biotrickling filters c | 1.480 | 0.010 |
| Absorber d | 2.334 | 0.018 |
| Adsorption | 1.200 | 0.009 |
| Desulphurisation Method | Annual Operating Cost (US $) a | ISBL Cost-Reference Capacity (US $) b | Investment Cost, for Reference Biogas Capacity in Year 2015 (US $) c | Investment Cost for New Biogas Capacity for Year 2015 (US $) d | Annualised Capital Cost for Year 2019 (US $) | Unit Cleaning Cost (US $/m3-biogas) |
|---|---|---|---|---|---|---|
| In-situ chemical dosing | 72,000 | 0 | 0 | 0 | 0 | 0.0100 |
| In-situ microaeration | 24,488 | 69,670.95 | 142,825.46 | 521,309.35 | 9189.517 | 0.0161 |
| Desulphurisation Method | Annualised Capital Cost (US $) a | Annual Operating Cost a | Unit Desulphurisation Cost (US $/m3) |
|---|---|---|---|
| Chelating iron | 1,260,362.64 | 504,000 | 0.245 |
| Bioscrubbers | 1,186,223.66 | 144,000 | 0.185 |
| Biofilter | 667,250.81 | 216,000 | 0.123 |
| Biotrickling filters | 10,972,568.89 | 72,000 | 1.534 |
| Absorption | 17,304,037.70 | 129,600 | 2.421 |
| Adsorption | 8,896,677.48 | 64,800 | 1.245 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okoro, O.V.; Sun, Z. Desulphurisation of Biogas: A Systematic Qualitative and Economic-Based Quantitative Review of Alternative Strategies. ChemEngineering 2019, 3, 76. https://0-doi-org.brum.beds.ac.uk/10.3390/chemengineering3030076
Okoro OV, Sun Z. Desulphurisation of Biogas: A Systematic Qualitative and Economic-Based Quantitative Review of Alternative Strategies. ChemEngineering. 2019; 3(3):76. https://0-doi-org.brum.beds.ac.uk/10.3390/chemengineering3030076
Chicago/Turabian StyleOkoro, Oseweuba Valentine, and Zhifa Sun. 2019. "Desulphurisation of Biogas: A Systematic Qualitative and Economic-Based Quantitative Review of Alternative Strategies" ChemEngineering 3, no. 3: 76. https://0-doi-org.brum.beds.ac.uk/10.3390/chemengineering3030076