Kinetic Studies of Cs+ and Sr2+ Ion Exchange Using Clinoptilolite in Static Columns and an Agitated Tubular Reactor (ATR)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Static Column Ion Exchange Experiments
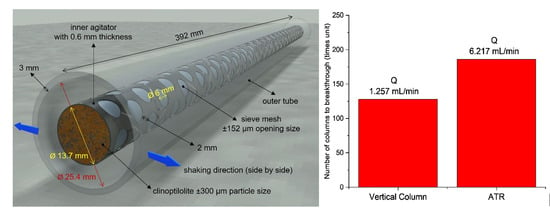
2.3. Agitated Tubular Reactor (ATR) Studies
2.4. Kinetics Breakthrough Model Analysis
3. Results and Discussion
3.1. Static Column Studies
3.2. Ion Exchange Performance Using an Agitated Tubular Reactor (ATR)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Budnitz, R.J. Strontium-90 and Strontium-89: A Review of Measurement Techniques in Environmental Media. In Proceedings of the 3rd InterAmerican Meeting on Radiochemistry, Sao Paulo, Brazil, 2–6 December 1974; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 1974; p. 15. [Google Scholar]
- Ojovan, M.I.; Lee, W.E. An Introduction to Nuclear Waste Immobilisation, 2nd ed.; Elsevier: Oxford, UK, 2014; pp. 75–97. [Google Scholar]
- Nagasaki, S.; Nakayama, S. Radioactive Waste Engineering and Management; Springer: Tokyo, Japan, 2015; pp. 17–46. [Google Scholar]
- De Haro-Del Rio, D.A.; Al-Jubouri, S.; Kontogiannis, O.; Papadatos-Gigantes, D.; Ajayi, O.; Li, C.; Holmes, S.M. The removal of caesium ions using supported clinoptilolite. J. Hazard. Mater. 2015, 289, 1–8. [Google Scholar] [CrossRef]
- Lehto, J.; Koivula, R.; Leinonen, H.; Tusa, E.; Harjula, R. Removal of Radionuclides from Fukushima Daiichi Waste Effluents. Sep. Purif. Rev. 2019, 48, 122–142. [Google Scholar] [CrossRef]
- Smičiklas, I.; Dimović, S.; Plećaš, I. Removal of Cs1+, Sr2+ and Co2+ from aqueous solutions by adsorption on natural clinoptilolite. Appl. Clay Sci. 2007, 35, 139–144. [Google Scholar] [CrossRef]
- Abusafa, A.; Yücel, H. Removal of 137 Cs from aqueous solutions using different cationic forms of a natural zeolite: Clinoptilolite. Sep. Purif. Technol. 2002, 28, 103–116. [Google Scholar] [CrossRef]
- Olatunji, M.A.; Khandaker, M.U.; Mahmud, H.N.M.E.; Amin, Y.M. Influence of adsorption parameters on cesium uptake from aqueous solutions—A brief review. RSC Adv. 2015, 5, 71658–71683. [Google Scholar] [CrossRef]
- Dyer, A.; Hriljac, J.; Evans, N.; Stokes, I.; Rand, P.; Kellet, S.; Harjula, R.; Moller, T.; Maher, Z.; Heatlie-Branson, R. The use of columns of the zeolite clinoptilolite in the remediation of aqueous nuclear waste streams. J. Radioanal. Nucl. Chem. 2018, 318, 2473–2491. [Google Scholar] [CrossRef] [Green Version]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gu, P.; Zhang, G.; Wang, X. Investigation of coagulation as a pretreatment for microfiltration in cesium removal by copper ferrocyanide adsorption. J. Radioanal. Nucl. Chem. 2017, 313, 435–444. [Google Scholar] [CrossRef]
- Ortiz-Oliveros, H.B.; Flores-Espinosa, R.M.; Jiménez-Domínguez, H.; Jiménez-Moleón, M.C.; Cruz-González, D. Dissolved air flotation for treating wastewater of the nuclear industry: Preliminary results. J. Radioanal. Nucl. Chem. 2012, 292, 957–965. [Google Scholar] [CrossRef]
- Ding, D.; Zhang, Z.; Chen, R.; Cai, T. Selective removal of cesium by ammonium molybdophosphate—Polyacrylonitrile bead and membrane. J. Hazard. Mater. 2017, 324, 753–761. [Google Scholar] [CrossRef]
- Aziz, M.; Beheir, S.G. Removal of 60Co and 134Cs from radioactive process waste water by flotation. J. Radioanal. Nucl. Chem. 1995, 191, 53–66. [Google Scholar] [CrossRef]
- Boglaienko, D.; Emerson, H.P.; Katsenovich, Y.P.; Levitskaia, T.G. Comparative analysis of ZVI materials for reductive separation of 99Tc(VII) from aqueous waste streams. J. Hazard. Mater. 2019, 380, 120836. [Google Scholar] [CrossRef]
- James, A.M.; Harding, S.; Robshaw, T.; Bramall, N.; Ogden, M.D.; Dawson, R. Selective Environmental Remediation of Strontium and Cesium Using Sulfonated Hyper-Cross-Linked Polymers (SHCPs). ACS Appl. Mater. Interfaces 2019, 11, 22464–22473. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Hodges, C.S.; Mishra, P.K.; Yoon, J.Y.; Hunter, T.N.; Lee, J.W.; Harbottle, D. Bio-Inspired Preparation of Clay–Hexacyanoferrate Composite Hydrogels as Super Adsorbents for Cs+. ACS Appl. Mater. Interfaces 2020, 12, 33173–33185. [Google Scholar] [CrossRef] [PubMed]
- Shiels, J.; Harbottle, D.; Hunter, T.N. Synthesis and physical property characterisation of spheroidal and cuboidal nuclear waste simulant dispersions. Materials 2018, 11, 1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awual, M.R.; Yaita, T.; Kobayashi, T.; Shiwaku, H.; Suzuki, S. Improving cesium removal to clean-up the contaminated water using modified conjugate material. J. Environ. Chem. Eng. 2020, 8, 103684. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, Y.K.; Hunter, T.N.; Brown, A.P.; Lee, J.W.; Harbottle, D. Organically modified clay with potassium copper hexacyanoferrate for enhanced Cs+ adsorption capacity and selective recovery by flotation. J. Mater. Chem. A 2017, 5, 15130–15143. [Google Scholar] [CrossRef] [Green Version]
- Valcke, E.; Engels, B.; Cremers, A. The use of zeolites as amendments in radiocaesium-and radiostrontium-contaminated soils: A soil-chemical approach. Part I: Cs-K exchange in clinoptilolite and mordenite. Zeolites 1997, 18, 205–211. [Google Scholar] [CrossRef]
- Valcke, E.; Engels, B.; Cremers, A. The use of zeolites as amendments in radiocaesium-and radiostrontium-contaminated soils: A soil-chemical approach. Part II: Sr-Ca exchange in clinoptilolite, mordenite, and zeolite A. Zeolites 1997, 18, 212–217. [Google Scholar] [CrossRef]
- Faghihian, H.; Marageh, M.G.; Kazemian, H. The use of clinoptilolite and its sodium form for removal of radioactive cesium, and strontium from nuclear wastewater and Pb2+, Ni2+, Cd2+, Ba2+ from municipal wastewater. Appl. Radiat. Isot. 1999, 50, 655–660. [Google Scholar] [CrossRef]
- Figueiredo, B.R.; Cardoso, S.P.; Portugal, I.; Rocha, J.; Silva, C.M. Inorganic Ion Exchangers for Cesium Removal from Radioactive Wastewater. Sep. Purif. Rev. 2018, 47, 306–336. [Google Scholar] [CrossRef]
- Ararem, A.; Bouras, O.; Bouzidi, A. Batch and continuous fixed-bed column adsorption of Cs+ and Sr2+ onto montmorillonite–iron oxide composite: Comparative and competitive study. J. Radioanal. Nucl. Chem. 2013, 298, 537–545. [Google Scholar] [CrossRef]
- Prajitno, M.Y.; Harbottle, D.; Hondow, N.; Zhang, H.; Hunter, T.N. The effect of pre-activation and milling on improving natural clinoptilolite for ion exchange of cesium and strontium. J. Environ. Chem. Eng. 2020, 8, 102991. [Google Scholar] [CrossRef]
- Moraetis, D.; Christidis, G.E.; Perdikatsis, V. Ion exchange equilibrium and structural changes in clinoptilolite irradiated with β-and γ-radiation: Monovalent cations. Am. Mineral. 2007, 92, 1714–1730. [Google Scholar] [CrossRef]
- Brooks, K.P. Cesium Ion Exchange Using Actual Waste: Column Size Considerations; Pacific Northwest National Laboratory: Richland, WA, USA, 1996; p. 40. [Google Scholar]
- El-Kamash, A. Evaluation of zeolite A for the sorptive removal of Cs+ and Sr 2+ ions from aqueous solutions using batch and fixed bed column operations. J. Hazard. Mater. 2008, 151, 432–445. [Google Scholar] [CrossRef]
- Patel, H. Fixed-bed column adsorption study: A comprehensive review. Appl. Water Sci. 2019, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Martínez, R.; Olguín, M.; Solache-Ríos, M. Cesium sorption by clinoptilolite-rich tuffs in batch and fixed-bed systems. Desalination 2010, 258, 164–170. [Google Scholar] [CrossRef]
- Borai, E.H.; Harjula, R.; Malinen, L.; Paajanen, A. Efficient removal of cesium from low-level radioactive liquid waste using natural and impregnated zeolite minerals. J. Hazard. Mater. 2009, 172, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Coward, T.; Tribe, H.; Harvey, A.P. Opportunities for process intensification in the UK water industry: A review. J. Water Process Eng. 2018, 21, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Stankiewicz, A.I.; Moulijn, J.A. Process intensification: Transforming chemical engineering. Chem. Eng. Prog. 2000, 96, 22–34. [Google Scholar]
- Wang, H.; Mustaffar, A.; Phan, A.N.; Zivkovic, V.; Reay, D.; Law, R.; Boodhoo, K. A review of process intensification applied to solids handling. Chem. Eng. Process. Process Intensif. 2017, 118, 78–107. [Google Scholar] [CrossRef]
- Hunter, T.N.; Pugh, R.J.; Franks, G.V.; Jameson, G.J. The role of particles in stabilising foams and emulsions. Adv. Colloid Interface Sci. 2008, 137, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Prajitno, M.Y.; Tangparitkul, S.; Zhang, H.; Harbottle, D.; Hunter, T.N. The effect of cationic surfactants on improving natural clinoptilolite for the flotation of cesium. J. Hazard. Mater. 2021, 402, 123567. [Google Scholar] [CrossRef]
- Zhang, H.; Tangparitkul, S.; Hendry, B.; Harper, J.; Kim, Y.K.; Hunter, T.N.; Lee, J.W.; Harbottle, D. Selective separation of cesium contaminated clays from pristine clays by flotation. Chem. Eng. J. 2019, 355, 797–804. [Google Scholar] [CrossRef]
- Maciejewski, P.; Walkowiak, W.; Robak, W. Selective removal of Cs-137, Sr-90, Ba-133, Co-60 and Pb-210 radioisotopes with proton-ionizable lariat ethers in the ion flotation process. Radioisotopes 2005, 2, R1. [Google Scholar]
- Shakir, K.; Ghoneimy, H.F.; Beheir, S.G.; Refaat, M. Flotation of Cesium Coprecipitated with Nickel Hexacyanoferrate(II) from Aqueous Solutions and Radioactive Waste Simulants. Sep. Sci. Technol. 2007, 42, 1341–1365. [Google Scholar] [CrossRef]
- Shakir, K.; Sohsah, M.; Soliman, M. Removal of cesium from aqueous solutions and radioactive waste simulants by coprecipitate flotation. Sep. Purif. Technol. 2007, 54, 373–381. [Google Scholar] [CrossRef]
- Zouboulis, A.; Peleka, E.; Zamboulis, D.; Matis, K. Application of Flotation for the Separation of Metal-Loaded Resins. Sep. Sci. Technol. 2005, 40, 861–876. [Google Scholar] [CrossRef]
- Ortiz-Oliveros, H.B.; Flores-Espinosa, R.M. Design of a mobile dissolved air flotation system with high rate for the treatment of liquid radioactive waste. Process Saf. Environ. Prot. 2020, 144, 23–31. [Google Scholar] [CrossRef]
- Nagar, A.; Pradeep, T. Clean Water through Nanotechnology: Needs, Gaps, and Fulfillment. ACS Nano 2020, 14, 6420–6435. [Google Scholar] [CrossRef]
- Kheshti, Z.; Ghajar, K.A.; Moreno-Atanasio, R.; Neville, F.; Ghasemi, S. Investigating the high gradient magnetic separator function for highly efficient adsorption of lead salt onto magnetic mesoporous silica microspheres and adsorbent recycling. Chem. Eng. Process. Process Intensif. 2020, 148, 107770. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, S.-M.; Yoon, I.-H.; Kim, I. Application of polyethylenimine-coated magnetic nanocomposites for the selective separation of Cs-enriched clay particles from radioactive soil. RSC Adv. 2020, 10, 21822–21829. [Google Scholar] [CrossRef]
- Lodha, H.; Jachuck, R.; Singaram, S.S. Intensified Biodiesel Production Using a Rotating Tube Reactor. Energy Fuels 2012, 26, 7037–7040. [Google Scholar] [CrossRef]
- Modak, J.B.; Bhowal, A.; Datta, S. Experimental study and mathematical modeling of breakthrough curve in rotating packed bed. Chem. Eng. Process. Process Intensif. 2016, 99, 19–24. [Google Scholar] [CrossRef]
- Visscher, F.; van der Schaaf, J.; Nijhuis, T.A.; Schouten, J.C. Rotating reactors—A review. Chem. Eng. Res. Des. 2013, 91, 1923–1940. [Google Scholar] [CrossRef]
- Wu, Y.; Chang, C.-C.; Guan, C.-Y.; Chang, C.-C.; Li, J.-W.; Chang, C.-Y.; Yu, C.-P. Enhanced removal of ammonium from the aqueous solution using a high-gravity rotating packed bed loaded with clinoptilolite. Sep. Purif. Technol. 2019, 221, 378–384. [Google Scholar] [CrossRef]
- Ng, Y.-S.; Tan, Y.-T.; Chua, A.S.M.; Hashim, M.A.; Sen Gupta, B. Removal of nickel from water using rotating packed bed contactor: Parametric studies and mode of operations. J. Water Process Eng. 2020, 36, 101286. [Google Scholar] [CrossRef]
- Brunet, L.; Prat, L.; Casamatta, G.; Carvin, P. An Innovative Pulsed Column Applied to Solid-Liquid Contacting the BPC Column. Chem. Eng. Technol. 2006, 29, 729–735. [Google Scholar] [CrossRef]
- Charton, S.; Duhamet, J.; Borda, G.; Ode, D. Axial dispersion in pulsed disk and doughnut columns: A unified law. Chem. Eng. Sci. 2012, 75, 468–477. [Google Scholar] [CrossRef]
- Jones, E.; McClean, K.; Housden, S.; Gasparini, G.; Archer, I. Biocatalytic oxidase: Batch to continuous. Chem. Eng. Res. Des. 2012, 90, 726–731. [Google Scholar] [CrossRef]
- Derksen, J.J. Mixing in an agitated tubular reactor. Can. J. Chem. Eng. 2019, 97, 523–527. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Bayly, A.E.; Hassanpour, A.; Fairweather, M.; Muller, F. Flow behaviour of an agitated tubular reactor using a novel dynamic mesh based CFD model. Chem. Eng. Sci. 2020, 212, 115333. [Google Scholar] [CrossRef]
- He, Y.; Bayly, A.E.; Hassanpour, A.; Muller, F.; Wu, K.; Yang, D. A GPU-based coupled SPH-DEM method for particle-fluid flow with free surfaces. Powder Technol. 2018, 338, 548–562. [Google Scholar] [CrossRef]
- Thomas, H.C. Chromatography: A Problem in Kinetics. Ann. N. Y. Acad. Sci. 1948, 49, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.C. Heterogeneous Ion Exchange in a Flowing System. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Yan, G.; Viraraghavan, T.; Chen, M. A New Model for Heavy Metal Removal in a Biosorption Column. Adsorpt. Sci. Technol. 2001, 19, 25–43. [Google Scholar] [CrossRef]
- Endo, M.; Yoshikawa, E.; Muramatsu, N.; Takizawa, N.; Kawai, T.; Unuma, H.; Sasaki, A.; Masano, A.; Takeyama, Y.; Kahara, T. The removal of cesium ion with natural Itaya zeolite and the ion exchange characteristics. J. Chem. Technol. Biotechnol. 2013, 88, 1597–1602. [Google Scholar] [CrossRef]
- Rajec, P.; Domianová, K. Cesium exchange reaction on natural and modified clinoptilolite zeolites. J. Radioanal. Nucl. Chem. 2007, 275, 503–508. [Google Scholar] [CrossRef]
- Rajec, P.; Macášek, F.; Feder, M.; Misaelides, P.; Šamajová, E. Sorption of caesium and strontium on clinoptilolite-and mordenite-containing sedimentary rocks. J. Radioanal. Nucl. Chem. 1998, 229, 49–55. [Google Scholar] [CrossRef]
- Amanipour, S.; Faghihian, H. Potassium hexacyanoferrate–clinoptilolite adsorbent for removal of Cs+ and Sr2+ from aqueous solutions. Int. J. Environ. Stud. 2017, 74, 86–104. [Google Scholar] [CrossRef]
- Ames, L.L., Jr. Effect of base cation on the cesium kinetics of clinoptilolite. Am. Mineral. 1962, 47, 1310–1316. [Google Scholar]
- Dyer, A. Use of zeolites in the treatment of nuclear waste. Anal. Proc. 1993, 30, 190–191. [Google Scholar] [CrossRef] [Green Version]
- NDA. Ion Exchange Material (Clinoptilolite) and Sand; Nuclear Decommissioning Authority: Cumbria, UK, 2013. [Google Scholar]
- Xu, Z.; Cai, J.-G.; Pan, B.-C. Mathematically modeling fixed-bed adsorption in aqueous systems. J. Zheijang Univ. Sci. A 2013, 14, 155–176. [Google Scholar] [CrossRef] [Green Version]
- Valisko, M.; Boda, D.; Gillespie, D. Selective adsorption of ions with different diameter and valence at highly charged interfaces. J. Phys. Chem. C 2007, 111, 15575–15585. [Google Scholar] [CrossRef]
- Ersoy, B.; Çelik, M.S. Electrokinetic properties of clinoptilolite with mono-and multivalent electrolytes. Microporous Mesoporous Mater. 2002, 55, 305–312. [Google Scholar] [CrossRef]
- Woods, R.-M.; Gunter, M.E. Na-and Cs-exchange in a clinoptilolite-rich rock: Analysis of the outgoing cations in solution. Am. Mineral. 2001, 86, 424–430. [Google Scholar] [CrossRef]
- Nightingale, E., Jr. Phenomenological theory of ion solvation. Effective radii of hydrated ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Mihaly-Cozmuta, L.; Mihaly-Cozmuta, A.; Peter, A.; Nicula, C.; Tutu, H.; Silipas, D.; Indrea, E. Adsorption of heavy metal cations by Na-clinoptilolite: Equilibrium and selectivity studies. J. Environ. Manag. 2014, 137, 69–80. [Google Scholar] [CrossRef]
- Tansel, B.; Sager, J.; Rector, T.; Garland, J.; Strayer, R.F.; Levine, L.; Roberts, M.; Hummerick, M.; Bauer, J. Significance of hydrated radius and hydration shells on ionic permeability during nanofiltration in dead end and cross flow modes. Sep. Purif. Technol. 2006, 51, 40–47. [Google Scholar] [CrossRef]
- Ames, L.L., Jr. Characterization of a strontium-selective zeolite. Am. Mineral. 1962, 47, 1317–1326. [Google Scholar]
- Ararem, A.; Bouzidi, A.; Mohamedi, B.; Bouras, O. Modeling of fixed-bed adsorption of Cs+ and Sr2+ onto clay–iron oxide composite using artificial neural network and constant–pattern wave approach. J. Radioanal. Nucl. Chem. 2014, 301, 881–887. [Google Scholar] [CrossRef]
- Foster, R.I.; Amphlett, J.T.M.; Kim, K.-W.; Kerry, T.; Lee, K.; Sharrad, C.A. SOHIO process legacy waste treatment: Uranium recovery using ion exchange. J. Ind. Eng. Chem. 2020, 81, 144–152. [Google Scholar] [CrossRef]
- McCabe, D.J. Comprehensive Scale Testing of the Ion Exchange Removal of Cesium and Technetium from Hanford Tank Wastes. In Proceedings of the Waste Management 01, Tucson, AZ, USA, 25 February–1 March 2001. [Google Scholar]
- Sana, D.; Jalila, S. A comparative study of adsorption and regeneration with different agricultural wastes as adsorbents for the removal of methylene blue from aqueous solution. Chin. J. Chem. Eng. 2017, 25, 1282–1287. [Google Scholar] [CrossRef]
- Lee, C.-G.; Kim, J.-H.; Kang, J.-K.; Kim, S.-B.; Park, S.-J.; Lee, S.-H.; Choi, J.-W. Comparative analysis of fixed-bed sorption models using phosphate breakthrough curves in slag filter media. Desalination Water Treat. 2015, 55, 1795–1805. [Google Scholar] [CrossRef]






| Experimental Parameters | Thomas Model | MDR Model | |||||
|---|---|---|---|---|---|---|---|
| Diameter (cm) | C0 (ppm) | Q (mL/min) | KTH (mL/mg·min) | qe (mg/g) | R2 | qe (mg/g) | R2 |
| Cs+ (30 min residence time) | |||||||
| 1 | 200 | 0.157 | 0.002 | 171.90 | 0.912 | 170.44 | 0.941 |
| Sr2+ (30 min residence time) | |||||||
| 1 | 200 | 0.157 | 0.004 | 15.31 | 0.981 | 16.67 | 0.948 |
| 1 | 100 | 0.157 | 0.012 | 48.10 | 0.978 | 47.96 | 0.985 |
| Sr2+ (15 min residence time) | |||||||
| 1 | 100 | 0.314 | 0.016 | 13.97 | 0.925 | 13.15 | 0.962 |
| 2 | 100 | 1.257 | 0.014 | 17.83 | 0.954 | 16.51 | 0.968 |
| Experimental Parameters | Thomas Model | MDR Model | ||||
|---|---|---|---|---|---|---|
| Bed Depth (cm) | Q (mL/min) | KTH (mL/mg·min) | qe (mg/g) | R2 | qe (mg/g) | R2 |
| 25 | 7.102 | 0.094 | 10.24 | 0.993 | 10.20 | 0.957 |
| 34 | 6.217 | 0.039 | 21.68 | 0.987 | 21.32 | 0.939 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prajitno, M.Y.; Taufiqurrakhman, M.; Harbottle, D.; Hunter, T.N. Kinetic Studies of Cs+ and Sr2+ Ion Exchange Using Clinoptilolite in Static Columns and an Agitated Tubular Reactor (ATR). ChemEngineering 2021, 5, 9. https://0-doi-org.brum.beds.ac.uk/10.3390/chemengineering5010009
Prajitno MY, Taufiqurrakhman M, Harbottle D, Hunter TN. Kinetic Studies of Cs+ and Sr2+ Ion Exchange Using Clinoptilolite in Static Columns and an Agitated Tubular Reactor (ATR). ChemEngineering. 2021; 5(1):9. https://0-doi-org.brum.beds.ac.uk/10.3390/chemengineering5010009
Chicago/Turabian StylePrajitno, Muhammad Yusuf, Mohamad Taufiqurrakhman, David Harbottle, and Timothy N. Hunter. 2021. "Kinetic Studies of Cs+ and Sr2+ Ion Exchange Using Clinoptilolite in Static Columns and an Agitated Tubular Reactor (ATR)" ChemEngineering 5, no. 1: 9. https://0-doi-org.brum.beds.ac.uk/10.3390/chemengineering5010009