Chirality Transfer in a Calixarene-Based Directional Pseudorotaxane Complex
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Details
2.2. Synthesis and Characterization of Hexamethoxycalix[6]arene 3 and (α-Methyl-Benzyl)Benzylammonium Axle 2+ as Barfate Salt
2.3. Synthesis and Characterization of the Pseudo[2]Rotaxane (Endo-Chiral)-2+@3
2.4. Computational Details
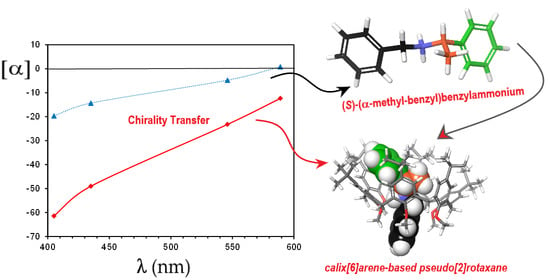
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jamieson, E.M.G.; Modicom, F.; Goldup, S.M. Chirality in rotaxanes and catenanes. Chem. Soc. Rev. 2018, 47, 5266–5311. [Google Scholar] [CrossRef] [Green Version]
- Evans, N.H. Chiral Catenanes and Rotaxanes: Fundamentals and Emerging Applications. Chem. Eur. J. 2018, 24, 3101–3112. [Google Scholar] [CrossRef]
- Hoekman, S.; Kitching, M.O.; Leigh, D.A.; Papmeyer, M.; Roke, D. Goldberg Active Template Synthesis of a [2]Rotaxane Ligand for Asymmetric Transition-Metal Catalysis. J. Am. Chem. Soc. 2015, 137, 7656–7659. [Google Scholar] [CrossRef] [PubMed]
- Heard, A.W.; Goldup, S.M. Synthesis of a Mechanically Planar Chiral Rotaxane Ligand for Enantioselective Catalysis. Chem 2020, 6, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, Y.; Erbas-Cakmak, S.; Leigh, D.A. Asymmetric Catalysis with a Mechanically Point-Chiral Rotaxane. J. Am. Chem. Soc. 2016, 138, 1749–1751. [Google Scholar] [CrossRef] [Green Version]
- Mitra, R.; Zhu, H.; Grimme, S.; Niemeyer, J. Functional Mechanically Interlocked Molecules: Asymmetric Organocatalysis with a Catenated Bifunctional Brønsted Acid. Angew. Chem. Int. Ed. 2017, 56, 11456–11459. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, Y.; Kihara, N.; Takata, T. Asymmetric benzoin condensation catalyzed by chiral rotaxanes tethering a thiazolium salt moiety via the cooperation of the component: Can rotaxane be an effective reaction field? J. Am. Chem. Soc. 2004, 126, 3438–3439. [Google Scholar] [CrossRef] [PubMed]
- Blanco, V.; Leigh, D.A.; Marcos, V.; Morales-Serna, J.A.; Nussbaumer, A.L. A Switchable [2]Rotaxane Asymmetric Organocatalyst That Utilizes an Acyclic Chiral Secondary Amine. J. Am. Chem. Soc. 2014, 136, 4905–4908. [Google Scholar] [CrossRef]
- Mitra, R.; Thiele, M.; Octa-Smolin, F.M.; Letzel, C.; Niemeyer, J. A bifunctional chiral [2]catenane based on 1,1’-binaphthyl-phosphates. Chem. Commun. 2016, 52, 5977–5980. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.Y.C.; Marques, I.; Félix, V.; Beer, P.D. Enantioselective Anion Recognition by Chiral Halogen-Bonding [2]Rotaxanes. J. Am. Chem. Soc. 2017, 139, 12228–12239. [Google Scholar] [CrossRef] [PubMed]
- Bruns, C.J.; Stoddart, J.F. The Nature of the Mechanical Bond: From Molecules to Machines, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Feringa, B.L. The art of building small: From molecular switches to motors. Angew. Chem. Int. Ed. 2017, 56, 11060–11078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauvage, J.-P. From chemical topology to molecular machines. Angew. Chem. Int. Ed. 2017, 56, 11080–11093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoddart, J.F. Mechanically interlocked molecules (MIMs)—Molecular shuttles, switches, and machines. Angew. Chem. Int. Ed. 2017, 56, 11094–11125. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, J.P.; Dietrich-Buchecker, C. Molecular Catenanes, Rotaxanes and Knots: A Journey through the World of Molecular Topology; Wiley-VCH: Weinheim, Germany, 1999. [Google Scholar]
- Xing, P.; Zhao, Y. Controlling supramolecular chirality in multicomponent self-assembled systems. Acc. Chem. Res. 2018, 51, 2324–2334. [Google Scholar] [CrossRef]
- Kuwahara, S.; Chamura, R.; Tsuchiya, S.; Ikeda, M.; Habata, Y. Chirality transcription and amplification by [2]pseudorotaxanes. Chem. Commun. 2013, 49, 2186–2188. [Google Scholar] [CrossRef]
- Wang, H.-J.; Zhang, H.-Y.; Zhang, H.-Y.; Liu, G.; Dai, X.; Wua, H.; Liu, Y. Guest-induced supramolecular chirality transfer in [2]pseudorotaxanes: Experimental and computational study. Org. Biomol. Chem. 2020, 18, 7649–7655. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; de Feyter, S. Amplification of chirality in surface-confined supramolecular bilayers. Nat. Commun. 2018, 9, 3416–3425. [Google Scholar] [CrossRef]
- Weiricha, L.; Merten, C. Induced VCD and conformational chirality in host–guest complexes of a chiral ammonium salt with crown ethers. Phys. Chem. Chem. Phys. 2021, 23, 18300–18307. [Google Scholar] [CrossRef]
- Asakawa, M.; Brancato, G.; Fanti, M.; Leigh, D.A.; Shimizu, T.; Slawin, A.M.Z.; Wong, J.K.Y.; Zerbetto, F.; Zhang, S. Switching “on” and “off” the expression of chirality in peptide rotaxanes. J. Am. Chem. Soc. 2002, 124, 2939–2950. [Google Scholar] [CrossRef]
- Saito, S.; Hirano, Y.; Mutoh, Y.; Kasama, T. Synthesis of a Homochiral [2]Rotaxane from a BINOL-derived Macrocyclic Phenanthroline. Chem. Lett. 2015, 44, 1509–1511. [Google Scholar] [CrossRef]
- Zhu, X.Z.; Chen, C.F. Efficient synthesis of a chiral [4]pseudocatenane and its derivatives: A novel ship’s wheel-like interlocked structure. Chem. Eur. J. 2006, 12, 5603–5609. [Google Scholar] [CrossRef]
- Gaeta, C.; Talotta, C.; Neri, P. Pseudorotaxane orientational stereoisomerism driven by π-electron density. Chem. Comm. 2014, 50, 9917–9920. [Google Scholar] [CrossRef]
- Gaeta, C.; Talotta, C.; Farina, F.; Camalli, M.; Campi, G.; Neri, P. Conformational features and recognition properties of a conformationally blocked calix[7]arene derivative. Chem. Eur. J. 2012, 18, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Della Sala, P.; Talotta, C.; Capobianco, A.; Soriente, A.; de Rosa, M.; Neri, P.; Gaeta, C. Synthesis, optoelectronic, and supramolecular properties of a calix[4]arene–cycloparaphenylene hybrid host. Org. Lett. 2018, 20, 7415–7418. [Google Scholar] [CrossRef] [PubMed]
- Talotta, C.; Gaeta, C.; Qi, Z.; Schalley, C.A.; Neri, P. Pseudorotaxanes with Self-Sorted Sequence and Stereochemical Orientation. Angew. Chem. Int. Ed. 2013, 52, 7437–7441. [Google Scholar] [CrossRef] [PubMed]
- Talotta, C.; Concilio, G.; Della Sala, P.; Gaeta, C.; Schalley, C.A.; Neri, P. Study on the influence of chirality in the threading of calix[6]arene hosts with dialkylammonium axles. Molecules 2020, 25, 5323. [Google Scholar] [CrossRef]
- Maier, J.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.; Simmerling, C. Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2005, 11, 3696–3713. [Google Scholar] [CrossRef] [Green Version]
- Krieger, E.; Vriend, G. YASARA view—Molecular graphics for all devices—From smartphones to workstations. Bioinformatics 2014, 30, 2981–2982. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision C.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Jurcik, A.; Bednar, D.; Byska, J.; Marques, S.M.; Furmanova, K.; Daniel, L.; Kokkonen, P.; Brezovsky, J.; Strnad, O.; Stourac, J.; et al. CAVER analyst 2.0: Analysis and visualization of channels and tunnels in protein structures and molecular dynamics trajectories. Bioinformatics 2018, 34, 3586–3588. [Google Scholar] [CrossRef] [Green Version]
- Arduini, A.; Orlandini, G.; Secchi, A.; Credi, A.; Silvi, S.; Venturi, M. Calixarene Threading by Viologen Axles. In Calixarenes and Beyond; Neri, P., Sessler, J.L., Wang, M.-X., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 761–781. [Google Scholar] [CrossRef]
- Schalley, C.A. Analytical Methods in Supramolecular Chemistry, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Suezawa, H.; Ishihara, S.; Umezawa, Y.; Tsuboyama, S.; Nishio, M. The aromatic CH/π hydrogen bond as an important factor in determining the relative stability of diastereomeric salts relevant to enantiomeric resolution—A crystallographic database study. Eur. J. Org. Chem. 2004, 2004, 4816–4822. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C.R. Valency and Bonding: A Natural Bond. Orbital Donor-Acceptor Perspective, 1st ed.; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talotta, C.; Concilio, G.; de Rosa, M.; Soriente, A.; Gaeta, C.; Rescifina, A.; Ballester, P.; Neri, P. Expanding coefficient: A parameter to assess the stability of induced-fit complexes. Org. Lett. 2021, 23, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- Vergura, S.; Scafato, P.; Belviso, S.; Superchi, S. Absolute configuration assignment from optical rotation data by means of biphenyl chiroptical probes. Chem. Eur. J. 2019, 25, 5682–5690. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, E.; Viglione, R.G.; Zanasi, R.; Rosini, C. Ab initio calculation of optical rotatory dispersion (ORD) curves: A simple and reliable approach to the assignment of the molecular absolute configuration. J. Am. Chem. Soc. 2004, 126, 12968–12976. [Google Scholar] [CrossRef]







| λ(nm) | [α]2+ b | αmix c | α2+ | α2+@3 | [α]2+@3 d |
|---|---|---|---|---|---|
| 589 | 0.8 | −0.030 | 0.0154 | −0.0316 | −12.4 |
| 546 | −4.8 | −0.0686 | −0.0922 | −0.0594 | −23.2 |
| 435 | −14.3 | −0.153 | −0.2746 | −0.1255 | −49.0 |
| 405 | −19.6 | −0.195 | −0.3763 | −0.1572 | −61.4 |
| φ and χ | Values (°) | Canting | Angles (θ) |
|---|---|---|---|
| χa φb | −78.72 | A | 75.35° |
| 106.08 | |||
| φa χb | 50.94 | B | 50.51° |
| −73.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Concilio, G.; Gaeta, C.; Della Sala, P.; Iuliano, V.; Talotta, C.; Monaco, G.; Superchi, S.; Belviso, S.; Neri, P. Chirality Transfer in a Calixarene-Based Directional Pseudorotaxane Complex. Chemistry 2021, 3, 1089-1100. https://0-doi-org.brum.beds.ac.uk/10.3390/chemistry3030079
Concilio G, Gaeta C, Della Sala P, Iuliano V, Talotta C, Monaco G, Superchi S, Belviso S, Neri P. Chirality Transfer in a Calixarene-Based Directional Pseudorotaxane Complex. Chemistry. 2021; 3(3):1089-1100. https://0-doi-org.brum.beds.ac.uk/10.3390/chemistry3030079
Chicago/Turabian StyleConcilio, Gerardo, Carmine Gaeta, Paolo Della Sala, Veronica Iuliano, Carmen Talotta, Guglielmo Monaco, Stefano Superchi, Sandra Belviso, and Placido Neri. 2021. "Chirality Transfer in a Calixarene-Based Directional Pseudorotaxane Complex" Chemistry 3, no. 3: 1089-1100. https://0-doi-org.brum.beds.ac.uk/10.3390/chemistry3030079