A Family of Externally-Functionalised Coordination Cages
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Details
2.2. X-ray Crystallography
3. Results
3.1. Reactions to Introduce 2-Acetyl Group onto Pyridine Nucleus
3.2. Preparation of LPEG and the Associated Cage HPEG
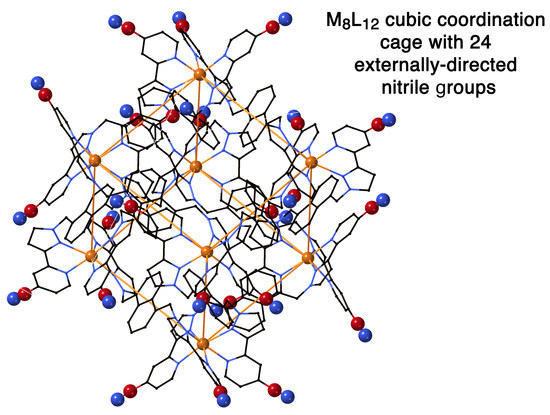
3.3. Preparation of LCN and the Associated Cage Cd•HCN; Crystal Structure of Cd•HCN
3.4. Preparation of LCC and the Associated Cage HCC; Crystal Structure of Ni•HCC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.; Ronson, T.K.; Nitschke, J.R. Functional capsules via subcomponent self-assembly. Acc. Chem. Res. 2018, 51, 2423–2436. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, M.; Klosterman, J.K.; Fujita, M. Functional molecular flasks: New properties and reactions within discrete, self-assembled hosts. Angew. Chem. Int. Ed. 2009, 48, 3418–3438. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, H.; Yusubov, M.; Verpoort, F. Self-assembled metal-organic polyhedra: An overview of various applications. Coord. Chem. Rev. 2016, 306, 171–194. [Google Scholar] [CrossRef]
- Cook, T.R.; Stang, P.J. Recent Developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular coordination: Self-assembly of finite two- and three-dimensional ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Stoddart, J.F. Emergent behaviour in nanoconfined molecular containers. Chem 2021, 7, 919–947. [Google Scholar] [CrossRef]
- Otte, M. Size-selective molecular flasks. ACS Catal. 2016, 6, 6491–6510. [Google Scholar] [CrossRef]
- Pullen, S.; Tessarolo, J.; Clever, G.H. Increasng structural and functional complexity in self-assembled cages. Chem. Sci. 2021, 12, 7269–7293. [Google Scholar] [CrossRef]
- Grommet, A.B.; Nitschke, J.R. Directed phase transfer of an FeII4L4 cage and encapsulated cargo. J. Am. Chem. Soc. 2017, 139, 2176–2179. [Google Scholar] [CrossRef] [Green Version]
- Garci, A.; Mbakidi, J.-P.; Chaleix, V.; Sol, V.; Orhan, E.; Therrien, B. Tunable arene ruthenium metallaprisms to transport, shield and release porphin in cancer cells. Organometallics 2015, 34, 4138–4146. [Google Scholar] [CrossRef]
- Mihara, N.; Ronson, T.K.; Nitschke, J.R. Different modes of anion response cause circulatory phase transfer of a coordination cage with controlled directionality. Angew. Chem. Int. Ed. 2019, 58, 12497–12501. [Google Scholar] [CrossRef]
- Grancha, T.; Carné-Sánchez, A.; Hernández-López, L.; Albalad, J.; Imaz, I.; Juanhuix, J.; Maspoch, D. Phase transfer of rhodium(II)-based metal-organic polyhedra bearing coordinatively bound cargo enables molecular separation. J. Am. Chem. Soc. 2019, 141, 18349–18355. [Google Scholar] [CrossRef]
- Jing, X.; He, C.; Zhao, L.; Duan, C. Photochemical properties of host-guest supramolecular systems with structurally confined metal-organic capsules. Acc. Chem. Res. 2019, 52, 100–109. [Google Scholar] [CrossRef]
- Gao, W.-X.; Zhang, H.-N.; Jin, G.-X. Supramolecular catalysis based on discrete heterometallic coordination-driven metallacycles and metallacages. Coord. Chem. Rev. 2019, 386, 69–84. [Google Scholar] [CrossRef]
- Brown, C.J.; Toste, F.D.; Bergman, R.G.; Raymond, K.N. Supramolecular catalysis in metal-ligand cluster hosts. Chem. Rev. 2015, 115, 3012–3025. [Google Scholar] [CrossRef]
- Fang, Y.; Powell, J.A.; Li, E.; Wang, Q.; Perry, Z.; Kirchon, A.; Yang, X.; Xiao, Z.; Zhu, C.; Zhang, L.; et al. Catalytic reactions within the cavity of coordination cages. Chem. Soc. Rev. 2019, 48, 4707–4730. [Google Scholar] [CrossRef]
- Severinson, R.J.; Rowlands, G.J.; Plieger, P.G. Coordination cages in catalysis. J. Incl. Phenom. Macrocycl. Chem. 2020, 96, 29–42. [Google Scholar] [CrossRef]
- Hong, C.M.; Bergman, R.G.; Raymond, K.N.; Toste, F.D. Self-assembled tetrahedral hosts as supramolecular catalysts. Acc. Chem. Res. 2018, 51, 2447–2455. [Google Scholar] [CrossRef]
- Olivio, G.; Capocasa, G.; Del Giudice, D.; Lanzalunga, O.; Di Stefano, S. New horizons for catalysis disclosed by supramolecular chemistry. Chem. Soc. Rev. 2021, 50, 7681–7724. [Google Scholar] [CrossRef]
- Xue, Y.; Hang, X.; Ding, J.; Li, B.; Zhu, R.; Pang, H.; Xu, Q. Catalysis within coordination cages. Coord. Chem. Rev. 2021, 430, 213656. [Google Scholar] [CrossRef]
- Gaeta, C.; La Manna, P.; De Rosa, M.; Soriente, A.; Talotta, C.; Neri, P. Supramolecular catalysis with self-assembled capsules and cages: What happens in confined spaces. ChemCatChem 2021, 13, 1638–1658. [Google Scholar] [CrossRef]
- Saha, M.L.; Yan, X.Z.; Stang, P.J. Photophysical properties of organoplatinum(II) compounds and derived self-assembled metallacycles and metallacages: Fluorescence and its applications. Acc. Chem. Res. 2016, 49, 2527–2539. [Google Scholar] [CrossRef]
- Fleming, J.S.; Mann, K.L.V.; Carraz, C.-A.; Psillakis, E.; Jeffery, J.C.; McCleverty, J.A.; Ward, M.D. Anion-templated assembly of a supramolecular cage complex. Angew. Chem. Int. Ed. Engl. 1998, 37, 1279–1281. [Google Scholar] [CrossRef]
- Metherell, A.J.; Cullen, W.; Williams, N.H.; Ward, M.D. Binding of hydrophobic guests in a coordination cage cavity is driven by liberation of ‘high-energy’ water. Chem. Eur. J. 2018, 24, 1554–1560. [Google Scholar] [CrossRef] [Green Version]
- Ronson, T.K.; Meng, W.J.; Nitschke, J.R. Design principles for the optimization of guest binding in aromatic-paneled FeII4L6 cages. J. Am. Chem. Soc. 2017, 139, 9698–9707. [Google Scholar] [CrossRef] [Green Version]
- Rizzuto, F.J.; Wood, D.M.; Ronson, T.K.; Mitschke, J.R. Tuning the redox properties of fullerene clusters within a metal-organic capsule. J. Am. Chem. Soc. 2017, 139, 11008–11011. [Google Scholar] [CrossRef] [Green Version]
- Mosquera, J.; Szyszko, B.; Ho, S.K.Y.; Nitschke, J.R. Sequence-selective encapsulation and protection of long peptides by a self-assembled FeII8L6 cubic cage. Nat. Commun. 2017, 8, 14882. [Google Scholar] [CrossRef]
- Tashiro, S.; Tominaga, M.; Kawano, M.; Therrien, B.; Ozeki, T.; Fujita, M. Sequence-selective recognition of peptides within the single binding pocket of a self-assembled coordination cage. J. Am. Chem. Soc. 2005, 127, 4546–4547. [Google Scholar] [CrossRef]
- Whitehead, M.; Turega, S.; Stephenson, A.; Hunter, C.A.; Ward, M.D. Quantification of solvent effects on molecular recognition in polyhedral coordination cage hosts. Chem. Sci. 2013, 4, 2744–2751. [Google Scholar] [CrossRef] [Green Version]
- Taggart, G.A.; Antonio, A.M.; Lorzing, G.R.; Yap, G.P.A.; Bloch, E.D. Tuning the porosity, solubility and gas-storage properties of cuboctahedral coordination cages via amide or ester functionalization. ACS Appl. Mater. Interfaces 2020, 12, 24913–24919. [Google Scholar] [CrossRef] [PubMed]
- Menozzi, E.; Pinalli, R.; Speets, E.A.; Ravoo, B.J.; Dalcanale, E.; Reinhoudt, D.N. Surface-confined single molecules: Assembly and disassembly of nanosize coordination cages on gold(111). Chem. Eur. J. 2004, 10, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Yoshimasa, Y.; Fujita, D.; Yagi-Utsumi, M.; Yamaguchi, T.; Kato, K.; Fujita, M. A self-assembled spherical complex displaying a gangliosidic glycan cluster capable of interacting with amyloidogenic proteins. Angew. Chem. Int. Ed. 2015, 54, 8435–8439. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, R.A.S.; Findlay, J.A.; Turner, D.R.; Crowley, J.D. Self-assembly of a redox-active, metallosupramolecular [Pd3L6]6+ complex using a rotationally flexible ferrocene ligand. Chem. Asian J. 2021, 16, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Neelakandan, P.P.; Jiménez, A.; Nitschke, J.R. Fluorophore incorporation allows nanomolar guest sensing and white-light emission in M4L6 cage complexes. Chem. Sci. 2014, 5, 908–915. [Google Scholar] [CrossRef]
- Tidmarsh, I.S.; Faust, T.B.; Adams, H.; Harding, L.P.; Russo, L.; Clegg, W.; Ward, M.D. Octanuclear cubic coordination cages. J. Am. Chem. Soc. 2008, 130, 15167–15175. [Google Scholar] [CrossRef]
- Ward, M.D.; Hunter, C.A.; Williams, N.H. Coordination cages based on bis(pyrazolylpyridine) ligands: Structures, dynamic behavior, guest binding, and catalysis. Acc. Chem. Res. 2018, 51, 2073–2082. [Google Scholar] [CrossRef] [Green Version]
- Turega, S.; Whitehead, M.; Hall, B.R.; Meijer, A.J.H.M.; Hunter, C.A.; Ward, M.D. Shape-, size- and functional group-selective binding of small organic guests in a paramagnetic coordination cage. Inorg. Chem. 2013, 52, 1122–1132. [Google Scholar] [CrossRef]
- Fontana, F.; Minisci, F.; Barbosa, M.C.N.; Vismara, E. Homolytic acylation of protonated pyridines and pyrazines with α-keto acids: The problem of monoacylation. J. Org. Chem. 1991, 56, 2866–2869. [Google Scholar] [CrossRef]
- Allan, D.R.; Nowell, H.; Barnett, S.A.; Warren, M.R.; Wilcox, A.; Christensen, J.; Saunders, L.K.; Peach, A.; Hooper, M.T.; Zaja, L.; et al. A novel dual air-bearing fixed-χ diffractometer for small-molecular single-crystal X-ray diffraction on beamline I-19 at Diamond Light Source. Crystals 2017, 7, 336. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.G.P.; Argent, S.P.; Ludden, M.D.; Piper, J.R.; Mozaceanu, C.; Barnett, S.A.; Ward, M.D. One guest or two? A crystallographic and solution study of guest binding in a cubic coordination cage. Chem. Eur. J. 2020, 26, 3054–3064. [Google Scholar] [CrossRef]
- Brunner, H.; Scheck, T. Neue optisch aktive Pyrazolderivate fur die enantioselektive Katalyse. Chem. Ber. 1992, 125, 701–709. [Google Scholar] [CrossRef]
- Lin, Y.; Lang, S.A. Novel two step synthesis of pyrazoles and isoxazoles from aryl methyl ketones. J. Heterocycl. Chem. 1977, 14, 345–347. [Google Scholar] [CrossRef]
- Björling, M.; Karlström, G.; Linse, P. Conformational adaption of poly(ethylene oxide): A carbon-13 NMR study. J. Phys. Chem. 1991, 95, 6706–6709. [Google Scholar] [CrossRef]
- Anderson, M.; Karlström, G. Conformational structure of 1,2-dimethoxyethane in water and other dipolar solvents, studied by quantum chemical, reaction field, and statistical mechanical techniques. J. Phys. Chem. 1985, 89, 4957–4962. [Google Scholar] [CrossRef]
- Piper, J.R.; Cletheroe, L.; Taylor, C.G.P.; Metherell, A.J.; Weinstein, J.A.; Sazanovich, I.V.; Ward, M.D. Photoinduced energy- and electron-transfer from a photoactive coordination cage to bound guests. Chem. Commun. 2017, 53, 408–411. [Google Scholar] [CrossRef] [Green Version]
- Train, J.S.; Wragg, A.B.; Auty, A.J.; Metherell, A.J.; Chekulaev, D.; Taylor, C.G.P.; Argent, S.P.; Weinstein, J.A.; Ward, M.D. Photophysics of cage/guest assemblies: Photoinduced electron transfer between a coordination cage containing osmium(II) luminophores, and electron-deficient bound guests in the central cavity. Inorg. Chem. 2019, 58, 2386–2396. [Google Scholar] [CrossRef]
- Aldhoun, M.; Massi, A.; Dondini, A. Click azide-nitrile cycloaddition as a new ligation tool for the synthesis of tetrazole-tethered C-glycosyl α-amino acids. J. Org. Chem. 2008, 73, 9565–9575. [Google Scholar] [CrossRef]
- Demko, Z.P.; Sharpless, K.B. A click chemistry approach to tetrazoles by Huisgen 1,3-dipolar cycloaddition: Synthesis of 5-acyltetrazoles from azides and acyl cyanides. Angew. Chem. Int. Ed. 2002, 41, 2113–2116. [Google Scholar] [CrossRef]
- Demko, Z.P.; Sharpless, K.B. A click chemistry approach to tetrazoles by Huisgen 1,3-dipolar cycloaddition: Synthesis of 5-sulfonyltetrazoles from azides and sulfonyl cyanides. Angew. Chem. Int. Ed. 2002, 41, 2110–2113. [Google Scholar] [CrossRef]
- Ludden, M.D.; Ward, M.D. Outside the box: Interactions of anions with the exterior surface of a cationic coordination cage. Dalton Trans. 2021, 50, 2782–2791. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Haldón, E.; Nicasio, M.C.; Pérez, P.J. Copper-catalysed azide-alkyne cycloaddition (CuAAC): An update. Org. Biomol. Chem. 2015, 13, 9528–9550. [Google Scholar] [CrossRef] [PubMed]
- Cullen, W.; Turega, S.; Hunter, C.A.; Ward, M.D. pH-Dependent binding of guests in the cavity of a polyhedral coordination cage: Reversible uptake and release of drug molecules. Chem. Sci. 2015, 6, 625–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Compound | Cd•HCN(•10.5MeCN) | Ni•HCC(•20DMF) |
|---|---|---|
| Empirical formula | B16C381Cd8F64H271.5N106.5 | B16C492F64H496N92Ni8O44 |
| Formula weight | 8629.69 | 9310.18 |
| T/K | 100(1) | 100(1) |
| Crystal system | Triclinic | Monoclinic |
| Space group | P–1 | P21/n |
| Crystal size / mm3 | 0.08 × 0.03 × 0.02 | 0.25 × 0.20 × 0.15 |
| a/Å | 21.1212(6) | 23.52269(18) |
| b/Å | 22.2410(9) | 46.1903(5) |
| c/Å | 24.6693(11) | 23.79481(19) |
| α/degrees | 116.279(3) | 90 |
| β/degrees | 98.215(3) | 101.6645(8) |
| γ/degrees | 101.119(3) | 90 |
| V/Å3 | 9846.7(4) | 25319.6(4) |
| Z | 1 | 2 |
| ρcalc/g cm−3 | 1.455 | 1.221 |
| μ/mm−1 | 0.485 | 1.064 |
| Radiation | Synchrotron (λ = 0.6889) | CuKα (λ = 1.54184) |
| Reflections collected | 122279 | 541416 |
| Data/restraints/parameters | 39594/8799/2566 | 54537/6902/2953 |
| Final R indexes [I ≥ 2σ(I)] | R1 = 0.0900, wR2 = 0.2744 | R1 = 0.1412, wR2 = 0.3935 |
| Final R indexes (all data) | R1 = 0.1528, wR2 = 0.3222 | R1 = 0.1694, wR2 = 0.4268 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, G.D.; Tipping, M.B.; Taylor, C.G.P.; Piper, J.R.; Pritchard, C.; Mozaceanu, C.; Ward, M.D. A Family of Externally-Functionalised Coordination Cages. Chemistry 2021, 3, 1203-1214. https://0-doi-org.brum.beds.ac.uk/10.3390/chemistry3040088
Jackson GD, Tipping MB, Taylor CGP, Piper JR, Pritchard C, Mozaceanu C, Ward MD. A Family of Externally-Functionalised Coordination Cages. Chemistry. 2021; 3(4):1203-1214. https://0-doi-org.brum.beds.ac.uk/10.3390/chemistry3040088
Chicago/Turabian StyleJackson, Garrett D., Max B. Tipping, Christopher G. P. Taylor, Jerico R. Piper, Callum Pritchard, Cristina Mozaceanu, and Michael D. Ward. 2021. "A Family of Externally-Functionalised Coordination Cages" Chemistry 3, no. 4: 1203-1214. https://0-doi-org.brum.beds.ac.uk/10.3390/chemistry3040088