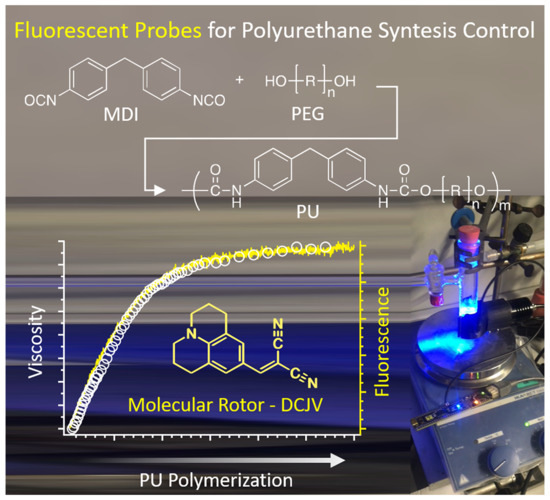
Molecular Rotors with Aggregation-Induced Emission (AIE) as Fluorescent Probes for the Control of Polyurethane Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymerization of MDI with PEG: Synthesis of PU and Viscosity Measurements
2.3. Polymerization of MDI with PEG: Synthesis of PU and Fluorescence Measurements
2.4. Polymerization of MDI with PEG: Synthesis of PU and Photodiode Measurements
2.5. Characterization and Instruments
2.6. Photodiode Set-Up
3. Results and Discussion
3.1. Characterization of the Prepared PU
3.2. Viscosity Measurements
3.3. Fluorescence Measurements
3.4. Use of the Photodiode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rigid Polyurethane (PU) Foams Market Analysis by Product and Segment Forecasts to 2020; Grand View Research: San Francisco, CA, USA, 2015.
- Bio-Based Polyurethane (PU) Market Analysis by Product and Segment Forecasts to 2020; Grand View Research: San Francisco, CA, USA, 2015.
- Adhesives and Sealants Market Size, Share & Trends Analysis Report by Technology, by Product, by Application, by Region (North America, Europe, Asia Pacific, CSA, MEA), and Segment Forecasts, 2019–2025; Grand View Research: San Francisco, CA, USA, 2019.
- Çatalgil-Giz, H.; Giz, A.; Alb, A.; Reed, W.F. Absolute online monitoring of a stepwise polymerization reaction: Polyurethane synthesis. J. Appl. Polym. Sci. 2001, 82, 2070–2077. [Google Scholar] [CrossRef]
- Wang, S.-K.; Sung, C.S.P. Fluorescence and IR Characterization of Cure in Polyurea, Polyurethane, and Polyurethane−Urea. Macromolecules 2002, 35, 883–888. [Google Scholar] [CrossRef]
- Peinado, C.; Salvador, E.F.; Baselga, J.; Catalina, F. Fluorescent Probes for Monitoring the UV Curing of Acrylic Adhesives, 1. FTIR and Fluorescence in Real Time. Macromol. Chem. Phys. 2001, 202, 1924–1934. [Google Scholar] [CrossRef] [Green Version]
- Hakala, K.; Vatanparast, R.; Li, S.; Peinado, C.; Bosch, P.; Catalina, F.; Lemmetyinen, H. Monitoring of Curing Process and Shelf Life of the Epoxy−Anhydride System with TICT Compounds by the Fluorescence Technique. Macromolecules 2000, 33, 5954–5959. [Google Scholar] [CrossRef]
- Bosch, P.; Fernández-Arizpe, A.; Catalina, F.; Mateo, J.L.; Peinado, C. New Fluorescent Probes for Monitoring Polymerization Reactions: Photocuring of Acrylic Adhesives, 2. Macromol. Chem. Phys. 2002, 203, 336–345. [Google Scholar] [CrossRef]
- González-Benito, J.; Mikeš, F.; Baselga, J.; Lemetyinemm, H. Fluorescence method using labeled chromophores to study the curing kinetics of a polyurethane system. J. Appl. Polym. Sci. 2002, 86, 2992–3000. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Wang, J.; Qiu, Z.; Zheng, X.; Leung, N.L.C.; Lam, J.W.Y.; Tang, B.Z. Multiscale Humidity Visualization by Environmentally Sensitive Fluorescent Molecular Rotors. Adv. Mater. 2017, 29, 1703900. [Google Scholar] [CrossRef]
- Haidekker, M.A.; Akers, W.; Lichlyter, D.; Brady, T.P.; Theodorakis, E.A. Sensing of Flow and Shear Stress Using Fluorescent Molecular Rotors. Sens. Lett. 2005, 3, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Haidekker, M.A.; Brady, T.P.; Lichlyter, D.; Theodorakis, E.A. Effects of solvent polarity and solvent viscosity on the fluorescent properties of molecular rotors and related probes. Bioorg. Chem. 2005, 33, 415–425. [Google Scholar] [CrossRef]
- Haidekker, M.A.; Theodorakis, E.A. Environment-sensitive behavior of fluorescent molecular rotors. J. Biol. Eng. 2010, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Mustafic, A.; Huang, H.-M.; Theodorakis, E.A.; Haidekker, M.A. Imaging of Flow Patterns with Fluorescent Molecular Rotors. J. Fluoresc. 2010, 20, 1087–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Shao, J.; Yang, Y.; Zhao, J.; Guo, H.; Li, X.; Ji, S.; Zhang, Z. Molecular Rotors as Fluorescent Viscosity Sensors: Molecular Design, Polarity Sensitivity, Dipole Moments Changes, Screening Solvents, and Deactivation Channel of the Excited States. Eur. J. Org. Chem. 2011, 2011, 4773–4787. [Google Scholar] [CrossRef]
- Guidugli, N.; Mori, R.; Bellina, F.; Tang, B.Z.; Pucci, A. Aggregation-Induced Emission: New Emerging Fluorophores for Environmental Sensing. In Principles and Applications of Aggregation-Induced Emission; Tang, Y., Tang, B.Z., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 335–349. [Google Scholar] [CrossRef]
- Sorgi, C.; Martinelli, E.; Galli, G.; Pucci, A. Julolidine-labelled fluorinated block copolymers for the development of two-layer films with highly sensitive vapochromic response. Sci. China Chem. 2018, 61, 947–956. [Google Scholar] [CrossRef]
- Borelli, M.; Iasilli, G.; Minei, P.; Pucci, A. Fluorescent polystyrene films for the detection of volatile organic compounds using the twisted intramolecular charge transfer mechanism †. Molecules 2017, 22, 1306. [Google Scholar] [CrossRef] [PubMed]
- Minei, P.; Pucci, A. Fluorescent vapochromism in synthetic polymers. Polym. Int. 2016, 65, 609–620. [Google Scholar] [CrossRef]
- Minei, P.; Koenig, M.; Battisti, A.; Ahmad, M.; Barone, V.; Torres, T.; Guldi, D.M.; Brancato, G.; Bottari, G.; Pucci, A. Reversible vapochromic response of polymer films doped with a highly emissive molecular rotor. J. Mater. Chem. C 2014, 2, 9224–9232. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-C.; Lee, J.-A.; Kwon, O.P.; Heo, J.; Seo, Y.H.; Kim, S.; Woo, H.C.; Lee, C.-L.; Kim, S.; Kim, S. Fluorescent Molecular Rotors for Viscosity Sensors. Chemistry 2018, 24, 13706–13718. [Google Scholar] [CrossRef]
- Haidekker, M.A.; Nipper, M.; Mustafic, A.; Lichlyter, D.; Dakanali, M.; Theodorakis, E.A. Dyes with segmental mobility: Molecular rotors. Springer Ser. Fluoresc. 2010, 8, 267–308. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef]
- Hu, R.; Leung, N.L.C.; Tang, B.Z. AIE macromolecules: Syntheses, structures and functionalities. Chem. Soc. Rev. 2014, 43, 4494–4562. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 4332–4353. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; You, J.; Fu, H.; Xue, T.; Hao, T.; Wang, X.; Wang, T. Photopolymerization with AIE dyes for solid-state luminophores. Polym. Chem. 2020, 11, 1589–1596. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, H.; Liu, S.; Sun, J.; Zhang, X.; Tang, B.Z. Polymerization-induced emission. Mater. Horiz. 2020, 7, 987–998. [Google Scholar] [CrossRef]
- Wang, X.; Qiao, X.; Yin, X.; Cui, Z.; Fu, P.; Liu, M.; Wang, G.; Pan, X.; Pang, X. Visualization of Atom Transfer Radical Polymerization by Aggregation-Induced Emission Technology. Chem. Asian J. 2020, 15, 1014–1017. [Google Scholar] [CrossRef]
- Li, K.; Qin, W.; Ding, D.; Tomczak, N.; Geng, J.; Liu, R.; Liu, J.; Zhang, X.; Liu, H.; Liu, B.; et al. Photostable fluorescent organic dots with aggregation-induced emission (AIE dots) for noninvasive long-term cell tracing. Sci. Rep. 2013, 3, 1150. [Google Scholar] [CrossRef]
- Król, P. Linear Polyurethanes: Synthesis Methods, Chemical Structures, Properties and Applications; Taylor & Francis: Abingdon, UK, 2008. [Google Scholar]
- Parnell, S.; Min, K.; Cakmak, M. Kinetic studies of polyurethane polymerization with Raman spectroscopy. Polymer 2003, 44, 5137–5144. [Google Scholar] [CrossRef]
- Verhoeven, V.W.A.; Padsalgikar, A.D.; Ganzeveld, K.J.; Janssen, L.P.B.M. A kinetic investigation of polyurethane polymerization for reactive extrusion purposes. J. Appl. Polym. Sci. 2006, 101, 370–382. [Google Scholar] [CrossRef] [Green Version]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minei, P.; Iasilli, G.; Ruggeri, G.; Mattoli, V.; Pucci, A. Molecular Rotors with Aggregation-Induced Emission (AIE) as Fluorescent Probes for the Control of Polyurethane Synthesis. Chemosensors 2021, 9, 3. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9010003
Minei P, Iasilli G, Ruggeri G, Mattoli V, Pucci A. Molecular Rotors with Aggregation-Induced Emission (AIE) as Fluorescent Probes for the Control of Polyurethane Synthesis. Chemosensors. 2021; 9(1):3. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9010003
Chicago/Turabian StyleMinei, Pierpaolo, Giuseppe Iasilli, Giacomo Ruggeri, Virgilio Mattoli, and Andrea Pucci. 2021. "Molecular Rotors with Aggregation-Induced Emission (AIE) as Fluorescent Probes for the Control of Polyurethane Synthesis" Chemosensors 9, no. 1: 3. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9010003