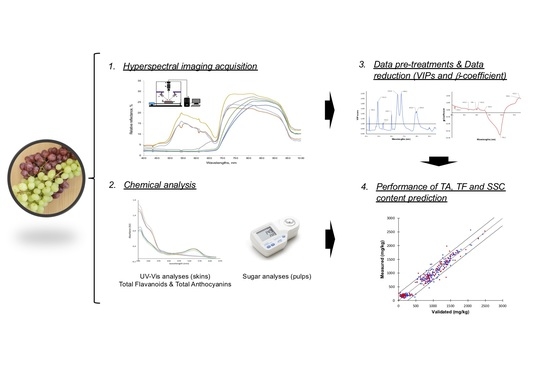
Hyperspectral Imaging to Characterize Table Grapes
Abstract
:1. Introduction
- I.
- Developing partial least square (PLS) models to validate the correlation between hyperspectral imaging spectra and Total Anthocyanins (TA) and Total Flavonoid (TF) contents and Total Soluble Solids (TSS), using the visible and short-wave near-infrared region;
- II.
- Selecting the lowest number of optimal wavelengths, based on regression coefficient (RC) and Variable Importance in Projection (VIPs) algorithms, which gave the highest correlation between the spectral data and the three selected quality parameters;
- III.
- Developing Multiple Regression Models (MLR) using spectra from only the optimal wavelengths and then checking the validation of the developed calibration models.
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Hyperspectral Imaging System (HIS)
2.4. Image Acquisition
2.5. Preprocessing of Hyperspectral Images
2.6. Data Analysis
2.6.1. Determination of Reference Parameters: Total Soluble Solids (TSS), Total Anthocyanin (TA), and Total Flavonoid Content (TF)
2.6.2. Spectral Analysis for Predicting Quality Attributes
- Collecting spectral data
- Spectra pre-treatments
2.6.3. Hyperspectral Imaging Calibration
- Model establishment
- Hyperspectral imaging model validation
- Hyperspectral imaging prediction
- Selection of optimal wavelengths
2.6.4. Statistical Analyses
3. Results
3.1. Grape Composition
3.2. Spectral Profiles
3.3. Modelization of Table Grape Composition Using the Whole Spectral Range of 411–1000 nm
3.4. Modelization of Table Grape Composition from Optimal Wavelengths Obtained by β-Coefficients
3.5. Modelization of Table Grape Composition from Optimal Wavelengths Obtained by VIPs Score
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Organisation of Vine and Wine (OIV). Statistical Report on World Vitiviniculture. 2019. Available online: http://www.oiv.int/public/medias/6782/oiv-2019-statistical-report-on-world-vitiviniculture.pdf (accessed on 12 January 2021).
- Valero, D.; Valverde, J.; Martínez-Romero, D.; Guillén, F.; Castillo, S.; Serrano, M. The combination of modified atmosphere packaging with eugenol or thymol to maintain quality, safety and functional properties of table grapes. Postharvest Biol. Technol. 2006, 41, 317–327. [Google Scholar] [CrossRef]
- Hellín, P.; Manso, A.; Flores, P.; Fenoll, J. Evolution of Aroma and Phenolic Compounds during Ripening of ’Superior Seedless’ Grapes. J. Agric. Food Chem. 2010, 58, 6334–6340. [Google Scholar] [CrossRef]
- Zeppa, G.; Rolle, L.; Gerbi, V. Valutazione mediante consumer test dell’attitudine al consumo diretto di un’uva a bacca rossa. Indus. Alim. 1999, 38, 818–824. [Google Scholar]
- Piva, C.R.; Garcia, J.L.L.; Morgan, W. The ideal table grapes for the Spanish market. Rev. Bras. Frutic. 2006, 28, 258–261. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, M.; Guan, L.; Lim, K.; Karim, A. The applications of computer vision system and tomographic radar imaging for assessing physical properties of food. J. Food Eng. 2004, 61, 125–135. [Google Scholar] [CrossRef]
- Turati, F.; Rossi, M.; Pelucchi, C.; Levi, F.; La Vecchia, C. Fruit and vegetables and cancer risk: A review of southern European studies. Br. J. Nutr. 2015, 113, S102–S110. [Google Scholar] [CrossRef]
- Vieira, A.R.; Abar, L.; Vingeliene, S.; Chan, D.S.M.; Aune, D.; Navarro-Rosenblatt, D.; Stevens, C.; Greenwood, D.; Norat, T. Fruits, vegetables and lung cancer risk: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 81–96. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Pereira, J.; Câmara, J.S. Healthy effects of bioactive metabolites from Vitis vinifera L. grapes: A review. In Grapes: Production, Phenolic Composition and Potential Biomedical Effects; Câmara, J.S., Ed.; Nova Science Technology: Hauppauge, NY, USA, 2014; pp. 305–338. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Basile, T.; Alba, V.; Gentilesco, G.; Savino, M.; Tarricone, L. Anthocyanins pattern variation in relation to thinning and girdling in commercial Sugrathirteen® table grape. Sci. Hortic. 2018, 227, 202–206. [Google Scholar] [CrossRef]
- Río Segade, S.; Giacosa, S.; Torchio, F.; De Palma, L.; Novello, V.; Gerbi, V.; Rolle, L. Impact of different advanced ripening stages on berry texture properties of ‘Red Globe’ and ‘Crimson Seedless’ table grape cultivars (Vitis vinifera L.). Sci. Hortic. 2013, 160, 313–319. [Google Scholar] [CrossRef]
- Kontoudakis, N.; Esteruelas, M.; Fort, F.; Canals, J.M.; Zamora, F. Comparison of methods for estimating phenolic maturity in grapes: Correlation between predicted and obtained parameters. Anal. Chim. Acta 2010, 660, 127–133. [Google Scholar] [CrossRef]
- Mota, A.; Pinto, J.; Fartouce, I.; Correia, M.J.; Costa, R.; Carvalho, R.; Aires, A.; Oliveira, A.A. Chemical profile and antioxidant potential of four table grape (Vitis vinifera) cultivars grown in Douro region, Portugal. Ciência Técnica Vitivinícola 2018, 33, 125–135. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, C.; Zhu, S.; Gao, P.; Feng, L.; He, Y. Non-Destructive and Rapid Variety Discrimination and Visualization of Single Grape Seed Using Near-Infrared Hyperspectral Imaging Technique and Multivariate Analysis. Molecules 2018, 23, 1352. [Google Scholar] [CrossRef] [Green Version]
- Fragoso, S.; Mestres, M.; Busto, O.; Guasch, J. Comparison of Three Extraction Methods Used to Evaluate Phenolic Ripening in Red Grapes. J. Agric. Food Chem. 2010, 58, 4071–4076. [Google Scholar] [CrossRef]
- Costa, G.; Noferini, M.; Fiori, G.; Torrigiani, P. Use of vis/nir spectroscopy to assess fruit ripening stage and improve management in post-harvest chain. Fresh Prod. 2009, 3, 35–41. [Google Scholar]
- Arendse, E.; Fawole, O.A.; Magwaza, L.S.; Opara, U.L. Non-destructive prediction of internal and external quality attributes of fruit with thick rind: A review. J. Food Eng. 2018, 217, 11–23. [Google Scholar] [CrossRef]
- Whitacre, E.; OLiver, J.; Broek, R.; Engelen, P.; Remers, B.K.; Horst, B.; Tewart, M.S.; Jansen-Beuvink, A. Predictive Analysis of Cocoa Procyanidins Using Near-Infrared Spectroscopy Techniques. J. Food Sci. 2003, 68, 2618–2622. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, J.; Liu, M.; Cai, J.; Liu, J. Determination of total polyphenols content in green tea using FT-NIR spectroscopy and different PLS algorithms. J. Pharm. Biomed. Anal. 2008, 46, 568–573. [Google Scholar] [CrossRef]
- Cozzolino, D.; Kwiatkowski, M.J.; Parker, M.; Cynkar, W.U.; Dambergs, R.G.; Gishen, M.; Herderich, M.J. Prediction of phenolic compounds in red wine fermentations by visible and near infrared spectroscopy. Anal. Chim. Acta 2004, 513, 73–80. [Google Scholar] [CrossRef]
- Versari, A.; Parpinello, G.P.; Mattioli, A.U.; Galassi, S. Determination of grape quality at harvest using Fourier-transform mid-infrared spectroscopy and multivariate analysis. Am. J. Enol. Vitic. 2008, 59, 317–322. [Google Scholar]
- Cozzolino, D.; Cynkar, W.U.; Dambergs, R.G.; Mercurio, M.D.; Smith, P.A. Measurement of Condensed Tannins and Dry Matter in Red Grape Homogenates Using Near Infrared Spectroscopy and Partial Least Squares. J. Agric. Food Chem. 2008, 56, 7631–7636. [Google Scholar] [CrossRef]
- Pathmanaban, P.; Gnanavel, B.; Anandan, S.S. Recent application of imaging techniques for fruit quality assessment. Trends Food Sci. Technol. 2019, 94, 32–42. [Google Scholar] [CrossRef]
- Ma, J.; Sun, D.-W.; Pu, H.; Cheng, J.-H.; Wei, Q. Advanced Techniques for Hyperspectral Imaging in the Food Industry: Principles and Recent Applications. Annu. Rev. Food Sci. Technol. 2019, 10, 197–220. [Google Scholar] [CrossRef] [PubMed]
- Cen, H.; Lu, R.; Ariana, D.P.; Mendoza, F. Hyperspectral imaging-based classification and wavebands selection for internal defect detection of pickling cucumbers. Food Bioproc. Technol. 2013, 7, 1689–1700. [Google Scholar] [CrossRef]
- Torres, I.; Amigo, J.-M. An overview of regression methods in hyperspectral and multispectral imaging. Data Handl. Sci. Technol. 2020, 32, 205–230. [Google Scholar] [CrossRef]
- Elmasry, G.; Kamruzzaman, M.; Sun, D.-W.; Allen, P. Principles and Applications of Hyperspectral Imaging in Quality Evaluation of Agro-Food Products: A Review. Crit. Rev. Food Sci. Nutr. 2012, 52, 999–1023. [Google Scholar] [CrossRef]
- Qin, J.; Kim, M.S.; Chao, K.; Chan, D.E.; Delwiche, S.R.; Cho, B.-K. Line-Scan Hyperspectral Imaging Techniques for Food Safety and Quality Applications. Appl. Sci. 2017, 7, 125. [Google Scholar] [CrossRef] [Green Version]
- Siche, R.; Vejarano, R.; Aredo, V.; Velasquez, L.; Saldaña, E.; Quevedo, R. Evaluation of Food Quality and Safety with Hyperspectral Imaging (HSI). Food Eng. Rev. 2016, 8, 306–322. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.-W. Advanced applications of hyperspectral imaging technology for food quality and safety analysis and assessment: A review—Part II: Applications. Innov. Food Sci. Emerg. Technol. 2013, 19, 15–28. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, S.; Zhang, C.; Bao, Y.; Gao, P.; He, Y. Variety Identification of Raisins Using Near-Infrared Hyperspectral Imaging. Molecules 2018, 23, 2907. [Google Scholar] [CrossRef] [Green Version]
- Calvini, R.; Ulrici, A.; Amigo, J.M. Practical comparison of sparse methods for classification of Arabica and Robusta coffee species using near infrared hyperspectral imaging. Chemom. Intell. Lab. Syst. 2015, 146, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekaran, I.; Panigrahi, S.S.; Ravikanth, L.; Singh, C.B. Potential of Near-Infrared (NIR) Spectroscopy and Hyperspectral Imaging for Quality and Safety Assessment of Fruits: An Overview. Food Anal. Methods 2019, 12, 2438–2458. [Google Scholar] [CrossRef]
- Pu, Y.-Y.; Feng, Y.-Z.; Sun, D.-W. Recent Progress of Hyperspectral Imaging on Quality and Safety Inspection of Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 176–188. [Google Scholar] [CrossRef]
- Gowen, A.; Odonnell, C.; Cullen, P.; Downey, G.; Frias, J. Hyperspectral imaging–an emerging process analytical tool for food quality and safety control. Trends Food Sci. Technol. 2007, 18, 590–598. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Gowen, A.A.; O’Donnell, C.P. Comparison of hyperspectral imaging with conventional RGB imaging for quality evaluation of Agaricus bisporus mushrooms. Biosyst. Eng. 2011, 108, 191–194. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Franco, C.; Mendes-Ferreira, A.; Mendes-Faia, A.; Da Costa, P.L.; Melo-Pinto, P. Brix, pH and anthocyanin content determination in whole Port wine grape berries by hyperspectral imaging and neural networks. Comput. Electron. Agric. 2015, 115, 88–96. [Google Scholar] [CrossRef]
- Tallada, J.G.; Bato, P.M.; Shrestha, B.P.; Kobayashi, T.; Nagata, M. Quality evaluation of plant products. In Hyperspectral Imaging Technology in Food and Agriculture; Park, B., Lu, R., Eds.; Springer: New York, NY, USA, 2015; pp. 227–249. [Google Scholar]
- Fracassetti, D.; Gabrielli, M.; Corona, O.; Tirelli, A. Characterisation of Vernaccia Nera (Vitis vinifera L.) grapes and wine. S. Afr. J. Enol. Vitic. 2017, 38, 72–81. [Google Scholar]
- Lee, J.; Kang, H. Flood fill mean shift: A robust segmentation algorithm. Int. J. Control. Autom. Syst. 2010, 8, 1313–1319. [Google Scholar] [CrossRef]
- Picouet, P.A.; Gou, P.; Hyypiö, R.; Castellari, M. Implementation of NIR technology for at-line rapid detection of sunflower oil adulterated with mineral oil. J. Food Eng. 2018, 230, 18–27. [Google Scholar] [CrossRef]
- Stchur, P.; Cleveland, D.; Zhou, J.; Michel, R.G. A review of recent applications of near infrared spectroscopy, and of the characteristics of a novel pbs ccd array-based near-infrared spectrometer. Appl. Spectrosc. Rev. 2002, 37, 383–428. [Google Scholar] [CrossRef]
- Rajkumar, P.; Wang, N.; Eimasry, G.; Raghavan, G.; Gariepy, Y. Studies on banana fruit quality and maturity stages using hyperspectral imaging. J. Food Eng. 2012, 108, 194–200. [Google Scholar] [CrossRef]
- Olah, M.; Bologa, C.; Oprea, T.I. An automated PLS search for biologically relevant QSAR descriptors. J. Comput. Mol. Des. 2004, 18, 437–449. [Google Scholar] [CrossRef] [Green Version]
- Mikulic-Petkovsek, M.; Skvarc, A.; Rusjan, D. Biochemical composition of different table grape cultivars produced in Slovenia. J. Hortic. Sci. Biotechnol. 2018, 94, 368–377. [Google Scholar] [CrossRef]
- Baiano, A.; Terracone, C.; Peri, G.; Romaniello, R. Application of hyperspectral imaging for prediction of physico-chemical and sensory characteristics of table grapes. Comput. Electron. Agric. 2012, 87, 142–151. [Google Scholar] [CrossRef]
- Costa, D.D.S.; Mesa, N.F.O.; Freire, M.S.; Ramos, R.P.; Mederos, B.J.T. Development of predictive models for quality and maturation stage attributes of wine grapes using vis-nir reflectance spectroscopy. Postharvest Biol. Technol. 2019, 150, 166–178. [Google Scholar] [CrossRef]
- Yang, Q.; Sun, D.-W.; Cheng, W. Development of simplified models for nondestructive hyperspectral imaging monitoring of TVB-N contents in cured meat during drying process. J. Food Eng. 2017, 192, 53–60. [Google Scholar] [CrossRef]
- De Marchi, M.; Fagan, C.; O’Donnell, C.; Cecchinato, A.; Zotto, R.D.; Cassandro, M.; Penasa, M.; Bittante, G. Prediction of coagulation properties, titratable acidity, and pH of bovine milk using mid-infrared spectroscopy. J. Dairy Sci. 2009, 92, 423–432. [Google Scholar] [CrossRef]
- Gherardi Hein, P.R.; Maioli Campos, A.C.; Trugilho, P.F.; Lima, J.T.; Chaix, G. Near infrared spectroscopy for estimating wood basic density in Eucalyptus urophylla and E. grandis. Cerne 2009, 15, 133–141. [Google Scholar]
- Pu, H.; Kamruzzaman, M.; Sun, D.-W. Selection of feature wavelengths for developing multispectral imaging systems for quality, safety and authenticity of muscle foods—A review. Trends Food Sci. Technol. 2015, 45, 86–104. [Google Scholar] [CrossRef]
- Galvão, R.K.H.; Araújo, M.C.U.; Fragoso, W.D.; Silva, E.C.; José, G.E.; Soares, S.F.C.; Paiva, H.M. A variable elimination method to improve the parsimony of MLR models using the successive projections algorithm. Chemom. Intell. Lab. Syst. 2008, 92, 83–91. [Google Scholar] [CrossRef]
- Andersen, C.M.; Bro, R. Variable selection in regression—A tutorial. J. Chemom. 2010, 24, 728–737. [Google Scholar] [CrossRef]
- Pu, H.; Liu, D.; Wang, L.; Sun, D.-W. Soluble Solids Content and pH Prediction and Maturity Discrimination of Lychee Fruits Using Visible and Near Infrared Hyperspectral Imaging. Food Anal. Methods 2016, 9, 235–244. [Google Scholar] [CrossRef]
- Gomes, V.M.; Fernandes, A.M.; Faia, A.; Melo-Pinto, P. Comparison of different approaches for the prediction of sugar content in new vintages of whole Port wine grape berries using hyperspectral imaging. Comput. Electron. Agric. 2017, 140, 244–254. [Google Scholar] [CrossRef]
- Nogales-Bueno, J.; Hernández-Hierro, J.M.; Rodríguez-Pulido, F.J.; Heredia, F.J. Determination of technological maturity of grapes and total phenolic compounds of grape skins in red and white cultivars during ripening by near infrared hyperspectral image: A preliminary approach. Food Chem. 2014, 152, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Piazzolla, F.; Amodio, M.L.; Colelli, G. Spectra evolution over on-vine holding of Italia table grapes: Prediction of maturity and discrimination for harvest times using a Vis-NIR hyperspectral device. J. Agric. Eng. 2017, 48, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Diago, M.P.; Fernández-Novales, J.; Fernandes, A.M.; Melo-Pinto, P.; Tardaguila, J. Use of Visible and Short-Wave Near-Infrared Hyperspectral Imaging to Fingerprint Anthocyanins in Intact Grape Berries. J. Agric. Food Chem. 2016, 64, 7658–7666. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, F.; Ning, J.; Liu, X.; Zhang, Z.; Yang, S. Predicting the anthocyanin content of wine grapes by NIR hyperspectral imaging. Food Chem. 2015, 172, 788–793. [Google Scholar] [CrossRef]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Rodríguez-Pulido, F.J.; Heredia, F.J.; Hernández-Hierro, J.M. Use of near infrared hyperspectral tools for the screening of extractable polyphenols in red grape skins. Food Chem. 2015, 172, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.M.; Oliveira, P.; Moura, J.P.; Oliveira, A.A.; Falco, V.; Correia, M.J.; Melo-Pinto, P. Determination of anthocyanin concentration in whole grape skins using hyperspectral imaging and adaptive boosting neural networks. J. Food Eng. 2011, 105, 216–226. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Fowler, M.S.; Fisk, I.D. Hyperspectral imaging for non-destructive prediction of fermentation index, polyphenol content and antioxidant activity in single cocoa beans. Food Chem. 2018, 258, 343–351. [Google Scholar] [CrossRef]
- Sen, I.; Ozturk, B.; Tokatli, F.; Ozen, B. Combination of visible and mid-infrared spectra for the prediction of chemical parameters of wines. Talanta 2016, 161, 130–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dambergs, R.; Gishen, M.; Cozzolino, D. A Review of the State of the Art, Limitations, and Perspectives of Infrared Spectroscopy for the Analysis of Wine Grapes, Must, and Grapevine Tissue. Appl. Spectrosc. Rev. 2015, 50, 261–278. [Google Scholar] [CrossRef]








| Grape Cultivars | Origin | TF (mg kg−1 F) | TA (mg kg−1 FM) | TSS (g 100 g−1) |
|---|---|---|---|---|
| Sable Seedless | South Africa | 1131 ± 267 c | 590 ± 163 a | 19.0 ± 1.8 b |
| Alphonse Lavallée | South Africa | 829 ± 153 d | 217 ± 61 c | 24.8 ± 1.1 a |
| Lival | France | 1642 ± 374 a | 588 ± 222 a | 15.0 ± 1.7 cd |
| Black Magic | Italy | 1279 ± 259 b | 399 ± 132 b | 15.4 ± 0.9 c |
| Sugarone Superior Seedless | South Africa | 162 ± 43 e | 0 | 14.7 ± 1.0 d |
| Thompson Seedless | Egypt | 826 ± 136 d | 0 | 15.5 ± 1.9 c |
| Victoria | Italy | 201 ± 28 e | 0 | 14.0 ± 1.5 e |
| p < 0.001 | p < 0.001 | p < 0.001 |
| Variable | Pre-Treatment | LVs | Calibration Set | Validation Set | Prediction Set | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2c | RMSEC | R2val | RMSEV | BIAS | RPD | R2pr | RMSEP | |||
| TF | SNV | 9 | 0.94 | 146 | 0.93 | 141 | −9.45 | 3.90 | 0.92 | 159 |
| 1st DER | 9 | 0.95 | 128 | 0.93 | 148 | 5.2 | 3.70 | 0.96 | 120 | |
| WD | 12 | 0.94 | 134 | 0.94 | 132 | −0.13 | 4.16 | 0.95 | 130 | |
| 2nd DER | 5 | 0.93 | 149 | 0.89 | 183 | 13.0 | 3.01 | 0.89 | 196 | |
| TA | SNV | 3 | 0.93 | 59 | 0.95 | 47 | 6.7 | 4.61 | 0.98 | 33 |
| 1st DER | 4 | 0.93 | 61 | 0.92 | 56 | 6.8 | 3.87 | 0.97 | 39 | |
| WD | 6 | 0.91 | 65 | 0.93 | 56 | 5.0 | 3.90 | 0.97 | 41 | |
| 2nd DER | 4 | 0.90 | 70 | 0.91 | 65 | 14.0 | 3.32 | 0.96 | 50 | |
| TSS | SNV | 10 | 0.94 | 1.0 | 0.91 | 1.1 | −0.05 | 3.45 | 0.95 | 0.9 |
| 1st DER | 6 | 0.93 | 1.0 | 0.91 | 1.2 | −0.07 | 3.33 | 0.93 | 1.1 | |
| WD | 15 | 0.96 | 0.8 | 0.94 | 0.9 | 0.01 | 4.17 | 0.96 | 0.8 | |
| 2nd DER | 5 | 0.92 | 1.1 | 0.88 | 1.4 | −0.01 | 2.90 | 0.92 | 1.1 | |
| Variable | Optimal Wavelengths (nm) | Calibration Set | Validation Set | Prediction Set | |||||
|---|---|---|---|---|---|---|---|---|---|
| R2c | RMSEC | R2val | RMSEV | Bias | RPD | R2pr | RMSEP | ||
| TF | 434.3, 485.5, 501.9, 543.4, 608.2, 631.4, 648.3, 675.9, 688.7, 707.9, 779, 792, 805, 807.2, 829, 905, 945.9 | 0.94 | 136 | 0.95 | 128 | 0.9 | 4.27 | 0.93 | 149 |
| TA | 434.3, 543.4, 604, 616.6, 669.5, 796.3, 943.6, 952.5 | 0.93 | 55 | 0.95 | 48 | 4.5 | 4.51 | 0.97 | 39 |
| TSS | 418, 434.3, 485, 501.9, 539.2, 543.4, 585.1, 646.2, 661, 678, 697.2, 716.5, 792, 802, 805, 807.2, 829, 833, 905.9, 910.3, 939.2, 945.9, 952.5 | 0.95 | 0.9 | 0.93 | 1.0 | −0.06 | 3.82 | 0.97 | 0.7 |
| Variable | Optimal Wavelengths (nm) | Calibration Set | Validation Set | Prediction Set | |||||
|---|---|---|---|---|---|---|---|---|---|
| R2c | RMSEC | R2val | RMSEV | Bias | RPD | R2pr | RMSEP | ||
| TF | 434.3, 543.4, 610.3, 633.5, 697.2, 781.1, 785.5, 805, 905.9, 910.3 | 0.90 | 178 | 0.90 | 178 | −11.3 | 3.09 | 0.93 | 155 |
| TA | 710, 785.5, 943.6 | 0.93 | 44 | 0.95 | 37 | 5.6 | 5.90 | 0.98 | 33 |
| TSS | 434.3, 501.9, 543.4, 610.3, 656.8, 686.5, 802.8, 809.4 | 0.86 | 1.5 | 0.83 | 1.6 | −0.06 | 2.46 | 0.86 | 1.4 |
| Variable | Spectral Windows (nm) | LVs | Calibration Set | Validation Set | Prediction Set | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2cal | RMSEC | R2val | RMSEV | Bias | RPD | R2pr | RMSEP | |||
| TF | 434.3, 539.2–543.4, 608.2–610.3, 620.8–639.8, 690.8–796.3, 829, 835.5–943.6 | 14 | 0.96 | 120 | 0.95 | 122 | 12.0 | 4.50 | 0.95 | 128 |
| TA | 697.2–802.8 and 842.1–957 | 8 | 0.95 | 38 | 0.96 | 33 | 1.5 | 6.50 | 0.99 | 27 |
| TSS | 420.1, 424.1, 428.2–432.3, 436.3, 479.3–481.4, 535.1–541.3, 545.4, 555.9, 560, 564.2, 585.1–639.8, 673.8–688.7, 716.5–720.8, 864, 881.6, 890.5–892.7, 899.3, 912.5–914.8, 921.4–934.7, 939.2, 954.8–957 | 14 | 0.94 | 1.0 | 0.89 | 1.3 | −0.01 | 3.00 | 0.94 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabrielli, M.; Lançon-Verdier, V.; Picouet, P.; Maury, C. Hyperspectral Imaging to Characterize Table Grapes. Chemosensors 2021, 9, 71. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9040071
Gabrielli M, Lançon-Verdier V, Picouet P, Maury C. Hyperspectral Imaging to Characterize Table Grapes. Chemosensors. 2021; 9(4):71. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9040071
Chicago/Turabian StyleGabrielli, Mario, Vanessa Lançon-Verdier, Pierre Picouet, and Chantal Maury. 2021. "Hyperspectral Imaging to Characterize Table Grapes" Chemosensors 9, no. 4: 71. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9040071