1. Introduction
The surface plasmon produced by the collective oscillation of conduction electrons in metal nanostructures can not only redistribute the electromagnetic field in time and space, but also redistributes the excited carriers. Various effects can be induced by the surface plasmons, including enhanced electromagnetic field, local heating, excited electrons, and excited holes that can drive many chemical reactions. Surface plasmons (SPS) originate from the collective oscillation of conduction electrons and play an important role in the optical properties of nanostructured metals and heavily doped semiconductors. Due to the deep understanding of SERS, when molecules, ions, free radicals, or other materials are adsorbed on, or near, some nanostructured metal surfaces, a significantly enhanced Raman scattering process will be generated. The application of SPS has been widely extended to spectroscopy, sensing, hyperthermia, waveguide, and so on [
1,
2,
3,
4,
5]. Now, it has been found that SPS can mediate some chemical processes by producing enhanced electromagnetic near field, excited carriers, or local heating effect. There are two main types of chemical reactions mediated by the plasma, including enhancement of the downhill reaction rate and inducing uphill reaction [
6,
7,
8,
9,
10].
SERS is a common spectral technique in which the inelastic light scattering of the molecules is greatly enhanced. SERS can enhance the weak Raman signal by 10
5–10
8, providing detailed structural information and binding properties of molecules on the surface [
4]. Since the discovery of SERS, the interest in SERS has been growing steadily and many other spectral techniques have been found. These techniques utilize the enhanced local field generated by the surface plasmon excitation to apply in optical phenomena, such as fluorescence or nonlinearity [
11,
12,
13]. In addition, the coupling of SERS with AFM or STM tips makes tip-enhanced Raman scattering a powerful imaging tool. For analytical applications, SERS provides abundant vibration spectrum information, which makes it different from many other technologies and achieves wide applications in many fields, such as electrochemistry, catalysis, biology, medicine, art protection, material science, etc [
14,
15,
16]. The enhancement mechanism of SERS has been always one of the hot topics in this field. The enhancement of Raman signal of probe molecules adsorbed on metal nanostructures comes from two factors, including the enhancement of electromagnetic field on the metal surface and increasing charge transfer between the metal and molecules. These two factors are attributed to the electromagnetic enhancement and chemical enhancement mechanisms, respectively [
17,
18,
19].
Plasma-driven surface catalytic oxidation-reduction reaction has attracted wide attention since it was first reported. From understanding its working principles to wide applications in physics, chemistry, environmental science, material science, and other fields, several reaction mechanisms of plasma-driven surface catalytic oxidation-reduction reaction are proposed and the plasma hot electrons produced through plasma decay played a key role, which not only provides electrons for reduction reaction but also provides a lot of kinetic energy for overcoming the potential energy barrier [
20,
21]. Recently, due to the great advantages of plasma exciton coupling interaction, the plasma exciton coupling co-driven surface catalytic reactions on the plasma metal graphene hybrid substrates are reported. The silver nanoparticles monolayer MoS
2 hybrid system has been also used in plasma exciton co-driven chemical reactions that significantly improved the probability and efficiency of the surface catalytic reactions. Plasma catalysis is an important result of local surface plasmon resonance of metal nanostructures. It has been proved that this method can be an effective route to convert light energy into chemical energy in CO
2 reduction, water decomposition, H
2 dissociation, or decomposition of organic pollutants [
22,
23,
24]. In order to characterize the plasma catalyst, dimerization of (PATP) to DMAB is studied. This is a typical model reaction and the reaction kinetics are observed by SERS [
25,
26].
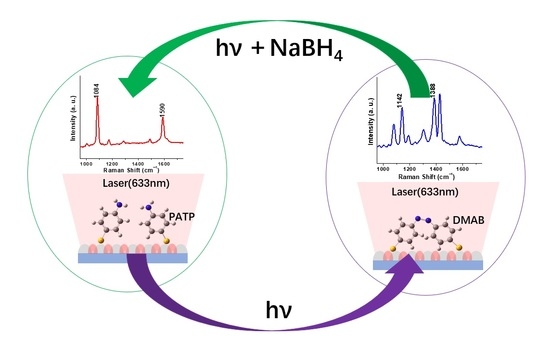
In this paper, a silver hemispherical nanoarray photocatalyst was first prepared with the help of an anodic aluminum oxide template. Using PATP as a probe molecule on the silver nanoarrays, the surface plasmon-driven photocatalytic reaction under 633 nm laser was studied by means of Raman spectroscopy. In addition, sodium borohydride was introduced in situ to realize the reverse photocatalytic reaction driven by the surface plasmon. The plasma distribution on the surface of the silver hemispherical nanoarray was simulated by the finite difference time domain method (FDTD) software. Through this method, it is expected to draw specific graphics or letters at the micro- and nano-scales, and realize the functions of graphics drawing, information encryption, reading, and erasing at these scales, which have significant practical applications.
3. Results and Discussion
This paper reports the photocatalytic reaction driven by plasma on the substrate of silver nanospherical arrays by the use of PATP as a probe molecule.
Figure 1a shows the Raman spectrum of PATP probe molecule. The black line is the Raman spectrum simulated by the Gaussian 09 software and the red line shows the Raman spectrum collected by the PATP solid powder excited at 633 nm. It can be seen from the figure that the theoretical and experimental values of the characteristic peaks of Raman spectrum corresponded well with each other. In the later stage, the PATP molecules were placed on the photocatalytic surface of silver nanospherical arrays. Due to the influence of the existing state of molecules and the surrounding environment of the silver surface, the peak position and relative intensity of Raman characteristic peaks will change. This phenomenon is a normal phenomenon shown by the surface enhanced Raman spectroscopy, which has no effect on the study of this paper. In this figure, the Raman characteristic peak of PATP molecule solid powder at 1084 cm
−1 corresponds to the 1080 cm
−1 Raman characteristic peak of this molecule on the surface of the silver nanoarray. Similarly,
Figure 1b shows the characteristic Raman peaks of DMAB molecules through experimental and theoretical calculations. By comparing
Figure 1a,b, it can be seen that the Raman peaks of PATP and DAMB at 1084 and 1142 cm
−1, respectively, do not overlap with each other. Therefore, in the following experiments, the Raman characteristic peaks at 1084 and 1142 cm
−1 can be corresponded to the existence of PATP and DMAB, respectively.
Figure 1d is a schematic diagram for the photocatalytic reaction process driven by the plasma on the surface of the silver nanospherical arrays. Firstly, the silver nanoarray substrate was immersed in the PATP solution, then the PATP molecules were evenly adsorbed on the surface of the silver ball. When the silver hemispherical nanoparticles showed a regular hexagonal arrangement on the substrate surface by the action of 633 nm laser, a very strong regular arrangement of local surface plasma enhanced hot spots were generated in the nanogap between the hemispherical particles. Compared to the excitation light itself, the intensity of the excited photoelectric magnetic field in the hot spot was greatly enhanced and the PATP probe molecule in the hot spot broke the nitrogen–hydrogen bond of the two molecules to form the nitrogen–nitrogen double bond under the dual action of surface plasma and excitation light, resulting in the photocatalytic reaction to form a new structure DMAB molecule. Then, by dropping 10
−2 mol/L sodium borohydride absolute ethanol solution in situ, the DMAB molecules located on the hemispherical surface of silver nanoparticles underwent reverse photocatalytic reaction under the action of surface plasma, excitation, and sodium borohydride to form the PATP molecules.
Figure 2 displays the AFM and SEM micrographs and morphology of the prepared spherical silver nanoarrays.
Figure 2a is a two-dimensional atomic force microscope picture of the silver nanoarray. It can be seen from the picture that the surface of the prepared spherical silver nanoarray is generally flat and each silver nanoparticle is hemispherical, uniform in size and regularly arranged. In addition, the same verification can be obtained from the SEM image of the silver nanoarray (
Figure 2b). The hemispherical nanoparticles generally show a regular hexagonal arrangement and the gap size between the particles is uniform. This regular hemispherical nanoarray structure has a large number of regularly arranged hot spots under the action of excitation. Through the linear section analysis of a group of array points in the two-dimensional picture of AFM (
Figure 2c), it can be further deduced that the diameter and height of each hemispherical silver nanoarray unit are relatively uniform. The diameter of each structural unit array was about 100 nm with the height of 20 nm. The gap between particle units was gradually declined with the decrease of height, and the middle size was about 50 nm. It can be seen from the comprehensive analysis in
Figure 2 that this regularly arranged silver nanoarray has a flat surface, uniform size, and monotonous distribution of the hot spots. It is an ideal catalytic substrate for the study of surface plasma-driven photocatalytic reaction.
However, does the PATP probe molecule located on the surface of the spherical silver nanoarray really have the photocatalytic ability and reverse photocatalytic reaction can occur under the action of 633 nm excitation in the presence of sodium borohydride, as shown in
Figure 1c? It can be verified by the real-time acquisition of Raman spectrum and identification of the characteristic peaks.
Figure 3 is the SERS spectra collected during the two-step stages of the photocatalytic reaction of PATP probe molecule based on the spherical silver nanoarray catalytic substrate under the action of 633 nm laser and reverse photocatalytic reaction caused by the in situ introduction of sodium borohydride.
Figure 3a shows the SERS spectra collected in real-time in the first stage. The four spectral lines in this figure from bottom to top are the SERS spectra collected every 2 s when the 633 nm laser is focused on the substrate surface under the condition of dark room. These spectral lines originated from continuous irradiation of the laser beam on the photocatalytic system for 2 s (a), 4 s (b), 6 s (c), and 8 s (d). According to the previous analysis, the SERS peaks at 1080 and 1142 cm
−1 can be attributed to the unique characteristic peaks of PATP and the photocatalytic product molecule DMAB, respectively. In
Figure 3a, the 1080 cm
−1 SERS characteristic peak represents the PATP molecule that always exists from the bottom to the top and proves the existence of the excessive PATP in the first stage of the photocatalytic process. The SERS peak at 1142 cm
−1 has appeared when the laser acted for 2 s and its intensity increases gradually with time. It can be seen that PATP molecules can undergo a very rapid photocatalytic reaction on the silver nanoarrays under the action of a 633 nm laser to produce DMAB molecules.
Figure 3b shows the changes of the integrated peak intensity ratio of 1142 and 1080 cm
−1 vibrations, corresponding to each spectral line in
Figure 3a. This intensity ratio can explain the change of DMAB content in the photocatalytic reaction to a certain extent.
Figure 3b explains that this ratio shows an obvious increasing trend with enhancing the illumination time. This finding shows that DMAB molecules were continuously and increasingly generated in the whole photocatalytic process.
Figure 3c shows the SERS spectra collected under the same experimental conditions after the first photocatalytic step by dropping sodium borohydride solution in situ.
Figure 3d depicts the changes of the integrated peak intensity ratio of 1142 and 1080 cm
−1 vibrations corresponding to each spectral line in
Figure 3c. From the bottom to the top of each spectral line, the variation trend of this integral peak intensity ratio in
Figure 3d is analyzed and compared. The 1080 cm
−1 SERS characteristic peak due to the PATP molecule always exists, which proves the existence of PATP in the whole second stage. Under the action of sodium borohydride and laser, the intensity of DMAB SERS peak at 1142 cm
−1 decreases rapidly with time until complete disappearance. At the same time, there is no new SERS peak, which means that no new molecules other than PATP and DMAB are produced in this process. It can be seen that the first stage of the photocatalytic production of DMAB molecule involves a reverse photocatalytic reaction under the action of sodium borohydride and laser to form PATP molecule. Through the analysis of
Figure 3, it can be concluded that the PATP probe molecule located on the surface of the spherical silver nanoarray undergoes a rapid photocatalytic reaction under the action of 633 nm excitation and hot spots on the silver nanoarray as well as DMAB product have been continuously and rapidly generated. Subsequently, DMAB molecules on the surface of the spherical silver nanoarray catalyzed the reverse photocatalytic reaction under the action of sodium borohydride, 633 nm excitation, and hot spots on the silver nanoarray to generate the PATP molecules again.
This photocatalysis driven by surface plasmon will realize the drawing and erasure of the molecular graphics on the nanoscale as well as the information encryption, reading, and erasure. The uniformly distributed probe molecule PATP was assembled on the surface of a specific catalytic substrate and irradiated with a focused laser beam with a certain wavelength to produce a new molecule DMAB. With the help of the micro- and nano-manipulation technology, the focused laser beam can be artificially controlled for the two-dimensional scanning on the catalytic substrate. Then, the PATP molecules in the region scanned by the excitation light undergo the photocatalytic reaction to generate DMAB. Noteworthy, the later molecule cannot be generated in the region not scanned. Therefore, the specific graphics or letters information can be drawn by DMAB molecular distribution on the micro- and nano-scales, thus, realizing micro- and nano-scale graphics drawing and information encryption. The two-dimensional imaging can be carried out with the characteristic peak intensity of DMAB molecules to display the drawn graphics and information decryption by using SERS spectrum scanning technology (mapping). In addition, sodium borohydride can be introduced into the encrypted substrate and the reverse photocatalytic reaction can occur under the action of surface plasma and stimulated luminescence, so as to realize the erasure of micro- and nano-scale graphics and encrypted information. To sum up, this will be a technical means with great scientific significance and practical value.
The surface plasma distribution characteristics on the substrate surface of the spherical silver nanoarray structure were calculated and simulated by the FDTD software (as shown in
Figure 4).
Figure 4a shows a theoretical model established according to the geometric dimensions of AFM diagrams of the prepared silver hemispherical nanoarray and the polarization direction of the stimulated luminescence stimulated by the silver nanoarray surface plasma.
Figure 4b shows the FDTD software calculation results of the surface plasma intensity distribution characteristics of the spherical silver nanoarrays. It can be seen from this figure that the strong local surface plasma enhancement hot spots are generated in the region between the spherical silver nanoparticles under the action of excitation. These hot spots are regularly arranged according to the distribution of particles and are dependent on the polarization direction of excitation light. In addition, the strong local surface plasmon enhancement around the silver nanoarray model is caused by the boundary effect in the simulation calculation. Due to the limitation of computing power, size of the nanoarray model is limited and cannot be as infinite as the actual periodic array substrate relative to the focused laser spot.
Figure 4c shows the variation of electromagnetic field intensity with X on the straight line at y = 0 in the calculation results. It can be seen from the figure that the electromagnetic field intensity on the surface of spherical silver nanoparticles is very low and the area close to the particle surface is very strong. At the same time, its intensity state represents a periodic distribution depending on the arrangement of hemispherical nanoparticles.
Figure 4d shows the changes of the surface plasma spatial distribution characteristics of spherical silver nanoarray with time after being affected by a stimulated luminescence plane wave. It can be seen that in the whole process after excitation, the local surface plasma oscillates between the spherical silver nanoparticle units and gradually decreases until disappearance.
Figure 4e is a schematic diagram of plasma-driven photocatalytic mechanism on the surface of the spherical silver nanoarrays. It can be seen from the figure that the energy required for PATP photocatalytic reaction mainly comes from three aspects. The first is that the PATP molecule is in contact with the spherical silver nanoarray, and its electron is affected by the silver Fermi level, which improves its energy. In the second part, the energy directly comes from the absorbed 633 nm excited light photons. The third part of the energy source is to absorb the energy of hot electrons and holes generated by the decay of surface plasma emitted by the spherical silver nanoarrays under the action of 633 nm laser.