1. Case Report
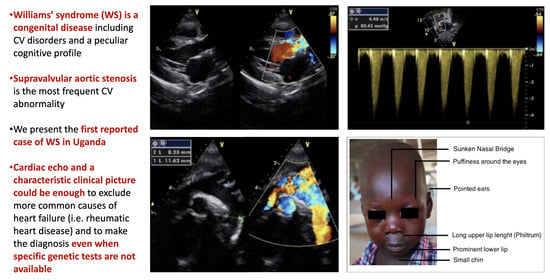
We report the case of a 5-year-old Ugandan child (O.B.) who was referred to a large nonprofit Ugandan hospital (Lacor Hospital, Gulu, North Uganda) for dyspnea and fever for two weeks with a referral diagnosis of “Rheumatic heart disease with superimposed endocarditis resulting in severe aortic stenosis”. On admission, the patient was febrile (37.5 °C) and sick looking with a dry cough and mild respiratory distress. On examination, the chest was clear with no signs of pulmonary congestion. Remarkably, the child had a characteristic facies with small chin, eyes puffiness, epicanthal folds and a long philtrum (
Figure 1). The weight was 13.5 Kg and height was 102 cm. No peripheral edema was noted. The patient was tachycardic with a regular heart rate of 115 beats per minute. The child’s blood pressure was 120/70 mmHg, which indicates hypertension for their age. He had a grade 4/6 ejection systolic murmur aortic valve area, radiating to the neck. The abdomen was soft without palpable masses.
We also noted features of moderate intellectual disability with poor short-term memory and a very limited vocabulary. He was also irritable and appeared restless at times. In addition, the patient showed signs of behavior uncommon among his peers. In particular, even though he was admitted to a hospital, he was remarkably sociable and prone to laughter and joking. Conversely, at times he would become frightened and burst into tears in response to modest stimuli (e.g., if a child near him raised his voice). In addition, the father reported that his son was used to react abnormally to intense noisy stimuli, such as a thunder during a thunderstorm, although this was a very frequent occurrence given the equatorial climate of the region (hypersensitivity to sounds). The rest of his neurologic physical exam was unremarkable. Specific neuropsychological tests were not conducted because these were not available in this setting.
A blood culture was negative and other laboratory findings, including full blood count, renal and liver function and electrolytes, were unremarkable. The ECG showed a sinus rhythm with LVH (
Figure 2). The cardiac echo showed a mild thickening of the aortic valve cusps without images suspected for vegetations and with a normal systolic motion. There was a discrete narrowing of ascending aorta (hourglass deformity) just above the sino-tubular junction. The ascending aorta diameter was 11.8 cm (Z-SCORE = 3.2 SD) (
Figure 3). The color-Doppler mapping showed a mosaic color pattern along the aortic root and proximal ascending aorta, while the continuous wave Doppler mode demonstrated a significant gradient (80 mmHg with a peak velocity of 4.5 m/s) consistent with a severe aortic stenosis (
Figure 4a,b). Left ventricle concentric hypertrophy was also noted: LVPWDd = 0.93 cm (Zscore = 3.98 SD). There was also turbulent flow across the pulmonary valve with mild valvular pulmonary stenosis (pressure gradient of 36 mmHg; peak velocity of 3 m/s). There was a post-stenotic dilatation of the main pulmonary artery. No other cardiac abnormalities were not noted. The aortic arch was normal (
Figure 5)
According to the echo findings and the clinical picture, a diagnosis of supravalvular aortic stenosis (SVAS) in Williams’ syndrome was made. The child was treated with HF medications, antibiotics for pneumonia and referred to a tertiary center with cardiac surgery at the capital for further management.
2. Discussion
In 1961, Williams described a group of four children with supravalvular aortic stenosis (SVAS) and a peculiar intellectual disability [
1]. Williams–Beuren syndrome (WS) is a rare (incidence is estimated at 1:25,000 live births) and complex developmental disorder including cardiovascular manifestations. Moreover, intellectual disability with a peculiar cognitive and/or behavior profile, a characteristic facies and occasional hypercalcemia are also described [
2].
From a genetic point of view, it is associated with a microdeletion in the 7q11.23 chromosomal region, which encompass the elastin gene. However, the proper pathogenic mechanism leading to the extensive vasculopathy is not entirely clear. In clinical studies, it has been shown that after 1 year, pulmonary arterial stenosis (PAS) tends to decline and SVAS worsens [
3,
4,
5]. The difficulty in the growth of the sino-tubular junction has been presented as a possible pathogenetic element to explain the progression of aortic disease [
5,
6].
The definite diagnosis should be made by the clinical picture assessed by a medical geneticist together with the demonstration of the typical elastin gene hemizygosity (assessed by FISH); however, genetic testing is often unavailable in developing countries. However, the combination of a typical clinical phenotype and echocardiographic profile could help to confirm the diagnosis.
In addition to the ascending aorta, stenosis may also occasionally occur in the aortic arc, the carotid and innominate arteries. Cardiovascular symptoms were previously reported in 47% of patients, while in a large series 77% were found to have a structural heart defect [
7]. SVAS was described as the most common diagnosis among children (79% of the cases). PAS is also common (41% of the subjects with cardiovascular manifestations). As a matter of fact, in our patient we observed a severe SVAS (peak gradient 80 mmHg) with normal anatomy and function of the aortic valve and mild valvular pulmonary stenosis, which are both fairly rare cardiovascular manifestations and compatible with the WS diagnosis.
In WS subjects, a characteristic so-called “elfin face” is also typical, consisting of a prominent metopic suture, small chin, sunken nasal bridge, eye puffiness, wide mouth, and prominent lower lip. This was also noted in our patient (
Figure 1).
During the neonatal period, cardiac manifestations are common in WS children and might help in making an early diagnosis when other features can remain unrecognized. On the other hand, a considerable percentage of the WS-associated cardiovascular problems may not manifest until adult age due to the fact that symptoms might be missing or not specific, delaying or preventing dedicated diagnostic procedures and proper treatments. A detailed cardiac evaluation must be performed in all WS patients due to the high prevalence of cardiovascular abnormalities [
8]. In addition to a diagnostic point of view, this is even more relevant to prognosis since when cardiac interventions are possible, the prognosis was relatively good and operative mortality low [
7].
3. Conclusions
We present the first reported case of Williams’ syndrome in Uganda. Cardiac echo and a characteristic clinical picture could be enough to exclude more common causes of heart failure (i.e., rheumatic heart disease) and to make a diagnosis even when specific genetic tests are not available.
Author Contributions
Conceptualization M.M. and P.Z.; Investigation M.M., A.T., V.C. and R.F.; Writing—original draft preparation, M.M., P.Z., R.F., P.A.; Writing—review and editing, M.M., V.C. and P.A.; visualization, M.M. and P.A.; supervision, P.A.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Ethical review and approval were waived for this study, due to its nature (single patient observational case report).
Informed Consent Statement
Written informed consent was obtained from the patient to publish this paper.
Data Availability Statement
No database is available due to the nature of the study (single patient observational case report).
Acknowledgments
This work is dedicated to the memory of Brother Elio Croce and his tireless work for Lacor Hospital. May he rest in peace.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Williams, J.C.; Barratt-Boyes, B.G.; Lowe, J.B. Supravalvular aortic stenosis. Circulation 1961, 24, 1311–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, C.A.; Demsey, S.A.; Leonard, C.O.; Dilts, C.; Blackburn, B.L. Natural history of Williams syndrome. J. Pediatr. 1988, 113, 318–326. [Google Scholar] [CrossRef]
- Keating, M.T. Genetic approaches to cardiovascular disease: Supravalvular aortic stenosis, Williams syndrome and long-QT syndrome. Circulation 1995, 92, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Faury, G.; Taylor, D.G.; Davis, E.C.; Boyle, W.A.; Mecham, R.P.; Stenzel, P.; Boak, B.; Keating, M.T. Novel arterial pathology in mice and humans hemizygous for elastin. J. Clin. Investig. 1998, 102, 1783–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.M.; Yoo, S.J.; Choi, J.Y.; Kim, S.H.; Bae, E.J.; Lee, Y.T. Natural course of supravalvular aortic stenosis and peripheral pulmonary stenosis in Williams syndrome. Cardiol. Young 1999, 9, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Wessel, A.; Pankau, R.; Kececioglu, D.; Ruschewski, W.; Bursch, J.H. Three decades of follow up of aortic and pulmonary vascular lesions in the Williams-Beuren syndrome. Am. J. Med. Genet. 1994, 52, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Eronen, M.; Peippo, M.; Hiippala, A.; Raatikka, M.; Arvio, M.; Johansson, R.; Kähkönen, M. Cardiovascular manifestations in 75 patients with Williams syndrome. J. Med. Genet. 2002, 39, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Sugayama, S.M.; Moisés, R.L.; Wagënfur, J.; Ikari, N.M.; Abe, K.T.; Leone, C.; Silva, C.A.A.D.; Chauffaille, M.D.L.L.F.; Chong, A.K. Williams-Beuren syndrome: Cardiovascular abnormalities in 20 patients diagnosed with fluorescence in situ hybridization. Arg. Bras. Cardiol. 2003, 81, 462–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).