Liver and Pancreatic Involvement in Children with Multisystem Inflammatory Syndrome Related to SARS-CoV-2: A Monocentric Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Procedures
2.3. Statistical Analysis
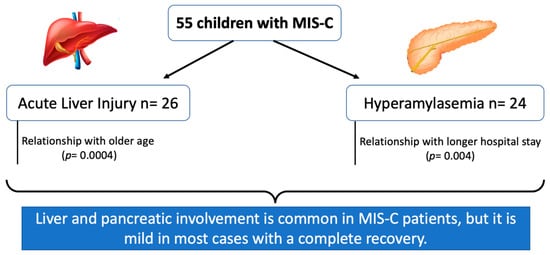
3. Results
3.1. Enrolled MIS-C Population
3.2. Liver Involvement
3.3. Pancreatic Involvement
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Center for Disease Control and Prevention. Information for Healthcare Providers about MultisystemInflammatory Syndrome in Children (MIS-C). Available online: https://www.cdc.gov/mis/mis-c/hcp/index.html (accessed on 18 April 2021).
- Giannattasio, A.; Maglione, M.; Zenzeri, L.; Mauro, A.; Di Mita, O.; Iodice, R.M.; Tipo, V. A child with a severe multisystem inflammatory syndrome following an asymptomatic COVID-19 infection: A novel management for a new disease? J. Med. Virol. 2021, 93, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J.; Fish, M.; Jennings, A.; Doores, K.J.; Wellman, P.; Seow, J.; Acors, S.; Graham, C.; Timms, E.; Kenny, J.; et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat. Med. 2020, 26, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Al-Beltagi, M.; Saeed, N.K.; Bediwy, A.S.; El-Sawaf, Y. Paediatric gastrointestinal disorders in SARS-CoV-2 infection: Epidemiological and clinical implications. World J. Gastroenterol. 2021, 27, 1716–1727. [Google Scholar] [CrossRef]
- Giannattasio, A.; D’Anna, C.; Muzzica, S.; Mauro, A.; Rosa, M.; Angrisani, M.; Acierno, S.; Savoia, F.; Tipo, V. Is COVID-19 a hyperferritinemic syndrome in children? Clin. Chem. Lab. Med. 2021, 59, e409–e412. [Google Scholar] [CrossRef] [PubMed]
- Bourkhissi, L.; El Fakiri, K.; Nassih, H.; El Qadiry, R.; Bourrahouat, A.; Sab, I.A.; Rada, N.; Draiss, G.; Bouskraoui, M. Laboratory abnormalities in children with novel Coronavirus Disease 2019. Clin. Med. Insights Pediatr. 2020, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Parri, N.; Lenge, M.; Buonsenso, D.; Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group. Children with Covid-19 in Pediatric Emergency Departments in Italy. N. Engl. J. Med. 2020, 383, 187–190. [Google Scholar] [CrossRef]
- Assa, A.; Benninga, M.A.; Borrelli, O.; Broekaert, I.; de Carpi, J.M.; Deganello Saccomani, M.; Dolinsek, J.; Mas, E.; Miele, E.; Thomson, M.; et al. Gastrointestinal Perspective of Coronavirus Disease 2019 in Children—An Updated Review. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 299–305. [Google Scholar] [CrossRef]
- Perez, A.; Cantor, A.; Rudolph, B.; Miller, J.; Kogan-Liberman, D.; Gao, Q.; Da Silva, B.; Margolis, K.G.; Ovchinsky, N.; Martinez, M. Liver involvement in children with SARS-CoV-2 infection: Two distinct clinical phenotypes caused by the same virus. Liver Int. 2021, 41, 2068–2075. [Google Scholar] [CrossRef]
- Kopiczko, N.; Kwiatek-Średzińska, K.; Uścinowicz, M.; Kowalczuk-Krystoń, M.; Lebensztejn, D.M. SARS-CoV-2 Infection as a Cause of Acute Pancreatitis in a Child—A Case Report. Pediatr. Rep. 2021, 13, 552–557. [Google Scholar] [CrossRef]
- Pegoraro, F.; Trapani, S.; Indolfi, G. Gastrointestinal, hepatic and pancreatic manifestations of COVID-19 in children. Clin. Res. Hepatol. Gastroenterol. 2021, 46, 101818. [Google Scholar] [CrossRef]
- Rowley, A.H. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat. Rev. Immunol. 2020, 20, 453–454. [Google Scholar] [CrossRef] [PubMed]
- Cantor, A.; Miller, J.; Zachariah, P.; DaSilva, B.; Margolis, K.; Martinez, M. Acute Hepatitis Is a Prominent Presentation of the Multisystem Inflammatory Syndrome in Children: A Single-Center Report. Hepatology 2020, 72, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Lazova, S.; Alexandrova, T.; Gorelyova-Stefanova, N.; Atanasov, K.; Tzotcheva, I.; Velikova, T. Liver Involvement in Children with COVID-19 and Multisystem Inflammatory Syndrome: A Single-Center Bulgarian Observational Study. Microorganisms 2021, 9, 1958. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Abu-El-Haija, M.; Kumar, S.; Quiros, J.A.; Balakrishnan, K.; Barth, B.; Bitton, S.; Eisses, J.F.; Foglio, E.J.; Fox, V.; Francis, D.; et al. Management of Acute Pancreatitis in the Pediatric Population: A Clinical Report From the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition Pancreas Committee. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.A.; Canna, S.W.; Friedman, K.G.; Gorelik, M.; Lapidus, S.K.; Bassiri, H.; Behrens, E.M.; Ferris, A.; Kernan, K.F.; Schulert, G.S.; et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated with SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 2. Arthritis Rheumatol. 2021, 73, e13–e29. [Google Scholar] [CrossRef]
- Cattalini, M.; Taddio, A.; Bracaglia, C.; Cimaz, R.; Paolera, S.D.; Filocamo, G.; La Torre, F.; Lattanzi, B.; Marchesi, A.; Simonini, G.; et al. Childhood multisystem inflammatory syndrome associated with COVID-19 (MIS-C): A diagnostic and treatment guidance from the Rheumatology Study Group of the Italian Society of Pediatrics. Ital. J. Pediatr. 2021, 47, 24. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, K.I.; Targher, G.; Byrne, C.D.; Zheng, M. Abnormal liver enzymes in children and infants with COVID-19: A narrative review of case-series studies. Pediatr. Obes. 2020, 15, e12723. [Google Scholar] [CrossRef]
- Jothimani, D.; Venugopal, R.; Abedin, M.F.; Kaliamoorthy, I.; Rela, M. COVID-19 and the liver. J. Hepatol. 2020, 73, 1231–1240. [Google Scholar] [CrossRef]
- Baroiu, L.; Dumitru, C.; Iancu, A.; Leșe, A.-C.; Drăgănescu, M.; Baroiu, N.; Anghel, L. COVID-19 impact on the liver. World J. Clin. Cases 2021, 9, 3814–3825. [Google Scholar] [CrossRef]
- Alqahtani, S.; Schattenberg, J.M. Liver injury in COVID-19: The current evidence. United Eur. Gastroenterol. J. 2020, 8, 509–519. [Google Scholar] [CrossRef]
- Effenberger, M.; Grander, C.; Grabherr, F.; Griesmacher, A.; Ploner, T.; Hartig, F.; Bellmann-Weiler, R.; Joannidis, M.; Zoller, H.; Weiss, G.; et al. Systemic inflammation as fuel for acute liver injury in COVID-19. Dig. Liver Dis. 2021, 53, 158–165. [Google Scholar] [CrossRef]
- Zhao, Y.; Patel, J.; Huang, Y.; Yin, L.; Tang, L. Cardiac markers of multisystem inflammatory syndrome in children (MIS-C) in COVID-19 patients: A meta-analysis. Am. J. Emerg. Med. 2021, 49, 62–70. [Google Scholar] [CrossRef]
- Palabiyik, F.; Akcay, N.; Sevketoglu, E.; Hatipoglu, N.; Sari, E.E.; Inci, E. Imaging of Multisystem Inflammatory Disease in Children (MIS-C) Associated With COVID-19. Acad. Radiol. 2021, 28, 1200–1208. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Tenforde, M.W.; Friedman, K.G.; Newhams, M.; Rose, E.B.; Dapul, H.; Soma, V.L.; Maddux, A.B.; Mourani, P.M.; Bowens, C.; et al. Overcoming COVID-19 Investigators. Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. JAMA 2021, 325, 1074–1087. [Google Scholar] [CrossRef]
- Acharyya, B.C.; Dutta, M.; Meur, S.; Das, D.; Acharyya, S. Acute Pancreatitis in COVID-19-associated Multisystem Inflammatory Syndrome of Children—A Single Center Experience. JPGN Rep. 2021, 3, e150. [Google Scholar] [CrossRef]
- Aslan, N.; Yildizdas, D.; Sinanoglu, M.S. A Pediatric COVID19 Case with Suspected Acute Abdomen, Hyperferritinemic Sepsis and Developing MIS-C and Pancreatitis. Indian J. Pediatr. 2021, 88, 288. [Google Scholar] [CrossRef]
- Stevens, J.P.; Brownell, J.N.; Freeman, A.J.; Bashaw, H. COVID-19-associated Multisystem Inflammatory Syndrome in Children Presenting as Acute Pancreatitis. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 669–671. [Google Scholar] [CrossRef]
- Vissers, R.J.; Abu-Laban, R.B.; McHugh, D.F. Amylase and lipase in the emergency department evaluation of acute pancreatitis. J. Emerg. Med. 1999, 17, 1027–1037. [Google Scholar] [CrossRef]
- Pribadi, R.R.; Simadibrata, M. Increased serum amylase and/or lipase in coronavirus disease 2019 (COVID-19) patients: Is it really pancreatic injury? JGH Open 2020, 5, 190–192. [Google Scholar] [CrossRef]
- De-Madaria, E.; Siau, K.; Cárdenas-Jaén, K. Increased Amylase and Lipase in Patients With COVID-19 Pneumonia: Don’t Blame the Pancreas Just Yet! Gastroenterology 2021, 160, 1871. [Google Scholar] [CrossRef]
- Vege, S.S. Approach to the Patient with Elevated Serum Amylase or Lipase. Uptodate. 2020. Available online: https://www.uptodate.com/contents/approach-to-the-patient-with-elevatedserum-amylase-or-lipase (accessed on 3 March 2022).
- Wang, F.; Wang, H.; Fan, J.; Zhang, Y.; Wang, H.; Zhao, Q. Pancreatic injury patterns in patients with COVID-19 pneumonia. Gastroenterology 2020, 159, 367–370. [Google Scholar] [CrossRef]
| Variable | n (%) |
|---|---|
| Males + | 29 (53) |
| Age (years) * | 6.5 ± 3.7 |
| Obesity + | 8 (14.5) |
| Duration of symptoms before hospital admission (days) * | 3.96 ± 2.12 |
| Clinical presentation + | |
| 55 (100) |
| 37 (67) |
| 30 (54) |
| 31 (56) |
| 28 (51) |
| 9 (16) |
| 3 (5) |
| No. of patients with ALI + | 16 (29) |
| No. of patients with raised pancreatic enzymes + | 5 (9) |
| Length of hospitalization (days) * | 13 ± 6 |
| Laboratory parameters: * | |
| 154.1 ± 87.7 |
| 9.17 ± 25.8 |
| 8.3 ± 8.8 |
| 832.2 ± 959.2 |
| 50 ± 55 |
| 49 ± 70 |
| 47 ± 48 |
| 38 ± 46 |
| Variables | ALI | Normal Transaminases | p |
|---|---|---|---|
| Number | 26 | 29 | |
| Males + | 9 (36) | 20 (69) | 0.02 |
| Age in years * | 8.3 ± 3.9 | 4.8 ± 2.7 | 0.0004 |
| Obesity + | 4 | 4 | ns |
| Duration of symptoms before hospital admission in days * | 4.23 ± 2 | 3.72 ± 2.2 | ns |
| Length of hospitalization in days * | 13.9 ± 6.2 | 11.9 ± 5.9 | ns |
| Inflammatory parameters *: | ns | ||
| 154.1 ± 87.7 | 138.7 ± 68.7 | |
| 13.7 ± 36.3 | 5.4 ± 8.4 | |
| 10.4 ± 7.3 | 6.4 ± 9.6 | |
| 916 ± 874.6 | 735.5 ± 1467.9 | |
| 88.9 ± 83 | 200.5 ± 287 | |
| 1995 ± 2988 | 1348 ± 1099 | |
| 641.4 ± 229 | 560 ± 191 | |
| 64 ± 63 | 32 ± 15 | 0.01 |
| 52 ± 55 | 19 ± 17 | ns |
| Troponin >ULN + | 17 (65) | 10 (34.5) | 0.02 |
| BNP * (pg/mL) (ref: <100) | 524 ± 1014 | 417 ± 652 | ns |
| Blood count: | ns | ||
| 10,895 ± 5878 | 12,231 ± 4582 | |
| 1519 ± 1627 | 2346 ± 2348 | |
| 199,923 ± 99,444 | 231,241 ± 93,986 |
| Variables | Raised Pancreatic Enzymes | Normal Pancreatic Enzymes | p |
|---|---|---|---|
| Number | 24 | 31 | |
| Males + | 10 (42%) | 19 (61%) | ns |
| Age in years * | 7.4 ± 3.5 | 5.7 ± 3.7 | ns |
| Obesity + | 4 | 4 | ns |
| Duration of symptoms before hospital admission (days) * | 4.13 ± 2.3 | 11.6 ± 2 | ns |
| Length of hospitalization (days) * | 14.7 ± 5.6 | 11.6 ± 6.1 | 0.004 |
| Inflammatory parameters: * | ns | ||
| 172.2 ± 8.2 | 139.3 ± 76.6 | |
| 7.2 ± 8.2 | 11 ± 33.7 | |
| 8.5 ± 6.8 | 8.2 ± 10.2 | |
| 886.8 ± 906.5 | 790.4 ± 1429.8 | |
| 145 ± 284 | 153 ± 155 | |
| 1932 ± 2816 | 1402 ± 1459 | |
| 650.5 ± 241.8 | 553.1 ± 172.4 | |
| 31 ± 12 | 43 ± 44 | ns |
| 47 ± 27 | 35 ± 32 | |
| Tryglicerides (mg/dL) | 203 ± 95 | 193 ± 159 | ns |
| Blood count: | ns | ||
| 11,076 ± 4583.6 | 12,036 ± 5752 | |
| 1910 ± 2482 | 1992 ± 1681 | |
| 222,120 ± 95,060 | 211,710 ± 94,439 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannattasio, A.; Maglione, M.; D’Anna, C.; Muzzica, S.; Pappacoda, S.; Lenta, S.; Di Mita, O.; Ranucci, G.; Mandato, C.; Tipo, V. Liver and Pancreatic Involvement in Children with Multisystem Inflammatory Syndrome Related to SARS-CoV-2: A Monocentric Study. Children 2022, 9, 575. https://0-doi-org.brum.beds.ac.uk/10.3390/children9040575
Giannattasio A, Maglione M, D’Anna C, Muzzica S, Pappacoda S, Lenta S, Di Mita O, Ranucci G, Mandato C, Tipo V. Liver and Pancreatic Involvement in Children with Multisystem Inflammatory Syndrome Related to SARS-CoV-2: A Monocentric Study. Children. 2022; 9(4):575. https://0-doi-org.brum.beds.ac.uk/10.3390/children9040575
Chicago/Turabian StyleGiannattasio, Antonietta, Marco Maglione, Carolina D’Anna, Stefania Muzzica, Serena Pappacoda, Selvaggia Lenta, Onorina Di Mita, Giusy Ranucci, Claudia Mandato, and Vincenzo Tipo. 2022. "Liver and Pancreatic Involvement in Children with Multisystem Inflammatory Syndrome Related to SARS-CoV-2: A Monocentric Study" Children 9, no. 4: 575. https://0-doi-org.brum.beds.ac.uk/10.3390/children9040575