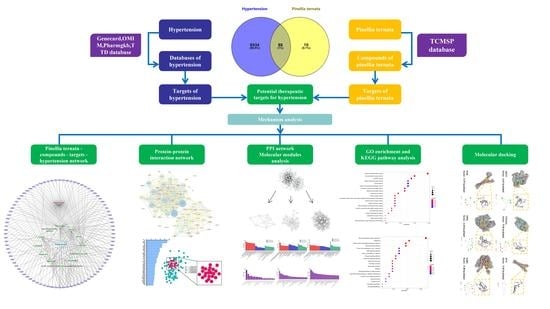
Network Pharmacology and Molecular Docking Combined to Analyze the Molecular and Pharmacological Mechanism of Pinellia ternata in the Treatment of Hypertension
Abstract
:1. Introduction
2. Materials and Methods
2.1. Screening of Effective Components of P. ternata and the Search for Its Action Target
2.1.1. Screening the Effective Components and Targets of P. ternata
2.1.2. Screening Targets for Hypertension in Humans
2.1.3. Compound-Target Network
2.2. Construction of Protein-Protein Interaction Network and Screening of Key Targets
2.2.1. Preliminary Construction of Protein-Protein Interaction Network
2.2.2. Structure Optimization of Interaction Network
2.2.3. Screening of Key Targets
2.3. Module Analysis of Protein-Protein Interaction Network
2.4. Go Functional Enrichment Analysis and KEGG Pathway Enrichment Analysis of Targets
2.5. Analysis of Molecular Docking and Action Forms of Effective Compounds
3. Results
3.1. Screen the Effective Compounds and Potential Targets of P. ternata
3.2. Compound-Target Network
3.3. Protein-Protein Interaction Network of Targets
3.4. Module Analysis of PPI Network
3.5. GO and KEGG Enrichment Analysis of Targets
3.6. Molecular Docking of Compounds and Targets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PPI | Protein-protein interaction |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| TCMSP | Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform |
| EPC | Edge Percolated Component |
| PDB | Protein Data Bank |
References
- Ventura, H.O.; Lavie, C.J. Editorial. Curr. Opin. Cardiol. 2019, 34, 329–330. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Elliott, W.J. Systemic Hypertension. Curr. Probl. Cardiol. 2007, 32, 201–259. [Google Scholar] [CrossRef] [PubMed]
- Di Palo, K.E.; Barone, N.J. Hypertension and Heart Failure. Heart Fail. Clin. 2020, 16, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Pistoia, F.; Sacco, S.; Degan, D.; Tiseo, C.; Ornello, R.; Carolei, A. Hypertension and Stroke: Epidemiological Aspects and Clinical Evaluation. High Blood Press. Cardiovasc. Prev. 2016, 23, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Lu, F.; Yang, A.; Dong, Y.; Liu, P.; Wang, Y. A Review on the Nonpharmacological Therapy of Traditional Chinese Medicine with Antihypertensive Effects. Evid. Based Complement. Altern. Med. 2019, 2019, 1317842. [Google Scholar] [CrossRef]
- Wenzel, U.O.; Bode, M.; Köhl, J.; Ehmke, H. A pathogenic role of complement in arterial hypertension and hypertensive end organ damage. Am. J. Physiol. Circ. Physiol. 2017, 312, H349–H354. [Google Scholar] [CrossRef] [Green Version]
- Schmieder, R.E. End Organ Damage in Hypertension. Dtsch. Aerzteblatt Online 2010, 107, 866–873. [Google Scholar] [CrossRef]
- Grassi, G. Sympathomodulatory Effects of Antihypertensive Drug Treatment. Am. J. Hypertens. 2016, 29, 665–675. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S. Antihypertensive drugs. Pharmacol. Res. 2017, 124, 116–125. [Google Scholar] [CrossRef]
- Stapff, M.; Hilderbrand, S. First-line treatment of essential hypertension: A real-world analysis across four antihypertensive treatment classes. J. Clin. Hypertens. 2019, 21, 627–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.; Kim, K.; Ihm, S.H.; Rhee, M.-Y.; Sohn, I.S.; Lee, H.-Y.; Park, S.; Jeon, E.-S.; Song, J.-M.; Pyun, W.B.; et al. Fimasartan versus perindopril with and without diuretics in the treatment of elderly patients with essential hypertension (Fimasartan in the Senior Subjects (FITNESS)): Study protocol for a randomized controlled trial. Trials 2019, 20, 389. [Google Scholar] [CrossRef]
- Blowey, D.L. Diuretics in the treatment of hypertension. Pediatr. Nephrol. 2016, 31, 2223–2233. [Google Scholar] [CrossRef] [PubMed]
- Rouzaud-Laborde, C.; Lafitte, P.; Balardy, L.; Czosnyka, Z.; Schmidt, E.A. ICP and Antihypertensive Drugs. In Acta Neurochirurgica, Supplementum; Springer: Cham, Switzerland, 2018; Volume 126, pp. 163–165. ISBN 9783319657981. [Google Scholar]
- Silva, I.V.G.; de Figueiredo, R.C.; Rios, D.R.A. Effect of Different Classes of Antihypertensive Drugs on Endothelial Function and Inflammation. Int. J. Mol. Sci. 2019, 20, 3458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wong, Y.-K.; Liao, F. What has traditional Chinese medicine delivered for modern medicine? Expert Rev. Mol. Med. 2018, 20, e4. [Google Scholar] [CrossRef]
- Chen, D.; Li, C.; Cai, H.; Zhuang, J.; Huang, Y.; Peng, X.; Li, S.; Huang, Y.; Wang, P.; Luo, Y.; et al. Effect of Banxia Baizhu Tianma Tang for H-type hypertension. Medicine 2020, 99, e19309. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, X.; Liu, W. Traditional Chinese Medicine Syndromes for Essential Hypertension: A Literature Analysis of 13,272 Patients. Evid. Based Complement. Altern. Med. 2014, 2014, 418206. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.-H.; Zhang, P.; Tao, Y.; Liu, Y.; Cao, G.; Zhou, L.; Yang, C.-H. Banxia Baizhu Tianma decoction attenuates obesity-related hypertension. J. Ethnopharmacol. 2021, 266, 113453. [Google Scholar] [CrossRef]
- Tan, C.S.; Loh, Y.C.; Ng, C.H.; Ch’ng, Y.S.; Asmawi, M.Z.; Ahmad, M.; Yam, M.F. Anti-hypertensive and vasodilatory effects of amended Banxia Baizhu Tianma Tang. Biomed. Pharmacother. 2018, 97, 985–994. [Google Scholar] [CrossRef]
- Luo, T.; Lu, Y.; Yan, S.; Xiao, X.; Rong, X.; Guo, J. Network Pharmacology in Research of Chinese Medicine Formula: Methodology, Application and Prospective. Chin. J. Integr. Med. 2020, 26, 72–80. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Schleinkofer, K.; Wang, T.; Wade, R.C. Molecular Docking. In Encyclopedic Reference of Genomics and Proteomics in Molecular Medicine; Springer: Berlin/Heidelberg, Germany, 2006; Volume 443, pp. 1149–1153. [Google Scholar]
- Saikia, S.; Bordoloi, M. Molecular Docking: Challenges, Advances and its Use in Drug Discovery Perspective. Curr. Drug Targets 2019, 20, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.-H.; Li, S.-M.; Xu, R.; Fang, L.; Xu, H.; Tong, P.-J. Predication of the underlying mechanism of Bushenhuoxue formula acting on knee osteoarthritis via network pharmacology-based analyses combined with experimental validation. J. Ethnopharmacol. 2020, 263, 113217. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Li, M.; Wang, J.; Pan, Y.; Wu, F.-X. CytoNCA: A cytoscape plugin for centrality analysis and evaluation of protein interaction networks. Biosystems 2015, 127, 67–72. [Google Scholar] [CrossRef]
- Bader, G.D.; Hogue, C.W.V. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Maere, S.; Heymans, K.; Kuiper, M. BiNGO: A Cytoscape plugin to assess overrepresentation of Gene Ontology categories in Biological Networks. Bioinformatics 2005, 21, 3448–3449. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [Green Version]
- Yu, G. ClusterProfiler: Universal enrichment tool for functional and comparative study. BioRxiv 2018. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Xu, Z. Using LeDock as a docking tool for computational drug design. IOP Conf. Ser. Earth Environ. Sci. 2019, 218, 012143. [Google Scholar] [CrossRef]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Prim. 2018, 4, 18014. [Google Scholar] [CrossRef] [Green Version]
- Eisele, N.; Albrecht, C.; Mistry, H.D.; Dick, B.; Baumann, M.; Surbek, D.; Currie, G.; Delles, C.; Mohaupt, M.G.; Escher, G.; et al. Placental expression of the angiogenic placental growth factor is stimulated by both aldosterone and simulated starvation. Placenta 2016, 40, 18–24. [Google Scholar] [CrossRef]
- Yang, G.; Sau, C.; Lai, W.; Cichon, J.; Li, W. Central Nervous System Neuroplasticity and the Sensitization of Hypertension. Nat. Rev. Nephrol. 2018, 14, 750–766. [Google Scholar] [CrossRef]
- Lopez Gelston, C.A.; Mitchell, B.M. Recent Advances in Immunity and Hypertension. Am. J. Hypertens. 2017, 30, 643–652. [Google Scholar] [CrossRef]
- Rucker, A.J.; Rudemiller, N.P.; Crowley, S.D. Salt, Hypertension, and Immunity. Annu. Rev. Physiol. 2018, 10, 283–307. [Google Scholar] [CrossRef]
- He, R.-L.; Wu, Z.-J.; Liu, X.-R.; Gui, L.-X.; Wang, R.-X.; Lin, M.-J. Calcineurin/NFAT Signaling Modulates Pulmonary Artery Smooth Muscle Cell Proliferation, Migration and Apoptosis in Monocrotaline-Induced Pulmonary Arterial Hypertension Rats. Cell. Physiol. Biochem. 2018, 49, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Tykocki, N.R.; Boerman, E.M.; Jackson, W.F. Smooth Muscle Ion Channels and Regulation of Vascular Tone in Resistance Arteries and Arterioles. In Comprehensive Physiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; Volume 7, pp. 485–581. [Google Scholar]
- Liu, Y.; Jiang, Y.; Li, W.; Han, C.; Zhou, L.; Hu, H. MicroRNA-200c-3p inhibits proliferation and migration of renal artery endothelial cells by directly targeting ZEB2. Exp. Cell Res. 2020, 387, 111778. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiong, X. Control Strategy on Hypertension in Chinese Medicine. Evid. Based Complement. Altern. Med. 2012, 2012, 284847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, X.; Yang, X.; Liu, W.; Feng, B.; Ma, J.; Du, X.; Wang, P.; Chu, F.; Li, J.; Wang, J. Banxia Baizhu Tianma Decoction for Essential Hypertension: A Systematic Review of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. 2012, 2012, 271462. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Yan, R.; Peng, J. Clinical Effect of Banxia Baizhu Tianma Decoction Combined with Western Medicine on Hypertension Xu Jiayang. Inn. Mong. J. Tradit. Chin. Med. 2019, 38, 54–55. [Google Scholar] [CrossRef]
- Tang, L.; Li, Z.; Bai, R. Study on the Mechanism of Banxia Baizhu Tianma Decoction on Essential Hypertension. World Chin. Med. 2020, 15, 2458–2465. [Google Scholar]
- Jiang, J.; Huang, D.; Li, Y.; Gan, Z.; Li, H.; Li, X.; Bian, K.; Ke, Y. Heart Protection by Herb Formula BanXia BaiZhu TianMa Decoction in Spontaneously Hypertensive Rats. Evid. Based Complement. Altern. Med. 2019, 2019, 5612929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Khalil, R.A. Matrix Metalloproteinases in Normal Pregnancy and Preeclampsia. Prog. Mol. Biol. Transl. Sci. 2017, 148, 87–165. [Google Scholar]
- Yung, H.; Calabrese, S.; Hynx, D.; Hemmings, B.A.; Cetin, I.; Charnock-Jones, D.S.; Burton, G.J. Evidence of Placental Translation Inhibition and Endoplasmic Reticulum Stress in the Etiology of Human Intrauterine Growth Restriction. Am. J. Pathol. 2008, 173, 451–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.; Park, K.; Lee, J.; Shin, Y.; An, S.; Kang, S.; Cho, W.; An, B.; Kim, S. The expression and activation of sex steroid receptors in the preeclamptic placenta. Int. J. Mol. Med. 2018, 41, 2943–2951. [Google Scholar] [CrossRef]
- Zhao, M.; Li, L.; Yang, X.; Cui, J.; Li, H. FN1, FOS, and ITGA5 induce preeclampsia: Abnormal expression and methylation. Hypertens. Pregnancy 2017, 36, 302–309. [Google Scholar] [CrossRef]
- Meeme, A.; Buga, G.A.; Mammen, M.; Namugowa, A. V Angiogenic imbalance as a contributor to the pathophysiology of preeclampsia among black African women. J. Matern. Neonatal Med. 2017, 30, 1335–1341. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Z.; Wang, C.; Xu, Q.; Liang, J.; Xu, X.; Yang, J.; Wang, C.; Jiang, T.; Yu, R. Application of reverse docking for target prediction of marine compounds with anti-tumor activity. J. Mol. Graph. Model. 2017, 77, 372–377. [Google Scholar] [CrossRef]





| Mol ID | Molecule Name | OB (%) | DL |
|---|---|---|---|
| MOL003578 | Cycloartenol | 38.69 | 0.78 |
| MOL001755 | 24-Ethylcholest-4-en-3-one | 36.08 | 0.76 |
| MOL000449 | Stigmasterol | 43.83 | 0.76 |
| MOL000358 | β-Sitosterol | 36.91 | 0.75 |
| MOL006936 | 10,13-Eicosadienoic | 39.99 | 0.2 |
| MOL005030 | Gondoic acid | 30.7 | 0.2 |
| MOL002670 | Cavidine | 35.64 | 0.81 |
| MOL002714 | Baicalein | 33.52 | 0.21 |
| MOL000519 | Coniferin | 31.11 | 0.32 |
| MOL006957 | (3S,6S)-3-(Benzyl)-6-(4-hydroxybenzyl) piperazine-2,5-quinone | 46.89 | 0.27 |
| MOL006937 | 12,13-Epoxy-9-hydroxynonadeca-7,10-dienoic acid | 42.15 | 0.24 |
| MOL002776 | Baicalin | 40.12 | 0.75 |
| MOL006967 | Xanthine-9β-D-ribofuranoside | 44.72 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Z.; Tao, X.; Alami, M.M.; Shu, S.; Wang, X. Network Pharmacology and Molecular Docking Combined to Analyze the Molecular and Pharmacological Mechanism of Pinellia ternata in the Treatment of Hypertension. Curr. Issues Mol. Biol. 2021, 43, 65-78. https://0-doi-org.brum.beds.ac.uk/10.3390/cimb43010006
Zhai Z, Tao X, Alami MM, Shu S, Wang X. Network Pharmacology and Molecular Docking Combined to Analyze the Molecular and Pharmacological Mechanism of Pinellia ternata in the Treatment of Hypertension. Current Issues in Molecular Biology. 2021; 43(1):65-78. https://0-doi-org.brum.beds.ac.uk/10.3390/cimb43010006
Chicago/Turabian StyleZhai, Zhaowei, Xinru Tao, Mohammad Murtaza Alami, Shaohua Shu, and Xuekui Wang. 2021. "Network Pharmacology and Molecular Docking Combined to Analyze the Molecular and Pharmacological Mechanism of Pinellia ternata in the Treatment of Hypertension" Current Issues in Molecular Biology 43, no. 1: 65-78. https://0-doi-org.brum.beds.ac.uk/10.3390/cimb43010006