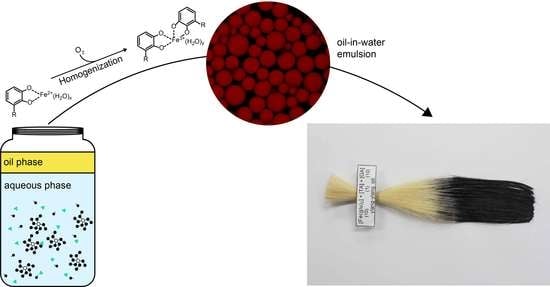
Iron Gall Ink Revisited: A Surfactant-Free Emulsion Technology for Black Hair-Dyeing Formulation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wood, J.M.; Decker, H.; Hartmann, H.; Chavan, B.; Rokos, H.; Spencer, J.D.; Hasse, S.; Thornton, M.J.; Shalbaf, M.; Paus, R.; et al. Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair. FASEB J. 2009, 23, 2065–2075. [Google Scholar] [CrossRef] [Green Version]
- A Shi, Y.; Luo, L.-F.; Liu, X.-M.; Zhou, Q.; Xu, S.-Z.; Lei, T.-C. Premature graying as a consequence of compromised antioxidant activity in hair bulb melanocytes and their precursors. PLoS ONE 2014, 9, e93589. [Google Scholar] [CrossRef] [Green Version]
- Trüeb, R.M. Oxidative stress in ageing of hair. Int. J. Trichology 2009, 1, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Arck, P.C.; Overall, R.; Spatz, K.; Liezman, C.; Handjiski, B.; Klapp, B.F.; Birch-Machin, M.A.; Peters, E.M.J. Towards a “free radical theory of graying”: Melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 2006, 20, 1567–1569. [Google Scholar] [CrossRef]
- Emerit, I.; Filipe, P.; Freitas, J.; Vassy, J. Protective effect of superoxide dismutase against hair graying in a mouse model. Photochem. Photobiol. 2004, 80, 579–582. [Google Scholar] [CrossRef]
- Irie, M.; Asami, S.; Nagata, S.; Miyata, M.; Kasai, H. Relationships between perceived workload, stress and oxidative DNA damage. Int. Arch. Occup. Environ. Health 2001, 74, 153–157. [Google Scholar] [CrossRef]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef] [Green Version]
- Akin Belli, A.; Etgu, F.; Ozbas Gok, S.; Kara, B.; Dogan, G. Risk factors for premature hair graying in young Turkish adults. Pediatr. Dermatol. 2016, 33, 438–442. [Google Scholar] [CrossRef]
- Shin, H.; Ryu, H.H.; Yoon, J.; Jo, S.; Jang, S.; Choi, M.; Kwon, O.; Jo, S.J. Association of premature hair graying with family history, smoking, and obesity: A cross-sectional study. J. Am. Acad. Dermatol. 2015, 72, 321–327. [Google Scholar] [CrossRef]
- Jo, S.J.; Paik, S.H.; Choi, J.W.; Lee, J.H.; Cho, S.; Kim, K.H.; Eun, H.C.; Kwon, O.S. Hair graying pattern depends on gender, onset age and smoking habits. Acta Derm. Venereol. 2012, 92, 160–161. [Google Scholar] [CrossRef] [Green Version]
- Trüeb, R.M. Association between smoking and hair loss: Another opportunity for health education against smoking? Dermatology 2003, 206, 189–191. [Google Scholar] [CrossRef]
- Kaur, K.; Kaur, R.; Bala, I. Therapeutics of premature hair graying: A long journey ahead. J. Cosmet. Dermatol. 2019, 18, 1206–1214. [Google Scholar] [CrossRef]
- Kumar, A.B.; Shamim, H.; Nagaraju, U. Premature graying of hair: Review with updates. Int. J. Trchology 2018, 10, 198–203. [Google Scholar] [CrossRef]
- Da França, S.A.; Dario, M.F.; Esteves, V.B.; Baby, A.R.; Velasco, M.V.R. Types of hair dye and their mechanisms of action. Cosmetics 2015, 2, 110–126. [Google Scholar] [CrossRef] [Green Version]
- Søsted, H.; Rustemeyer, T.; Gonçalo, M.; Bruze, M.; Goossens, A.; Giménez-Arnau, A.M.; Le Coz, C.J.; White, I.R.; Diepgen, T.L.; Andersen, K.E.; et al. Contact allergy to common ingredients in hair dyes. Contact Dermat. 2013, 69, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Takkouche, B.; Etminan, M.; Montes-Martínez, A. Personal use of hair dyes and risk of cancer: A meta-analysis. JAMA 2005, 293, 2516–2525. [Google Scholar] [CrossRef]
- Zhang, Y.; Sanjose, S.D.; Bracci, P.M.; Morton, L.M.; Wang, R.; Brennan, P.; Hartge, P.; Boffetta, P.; Becker, N.; Maynadie, M.; et al. Personal use of hair dye and the risk of certain subtypes of non-Hodgkin lymphoma. Am. J. Epidemiol. 2008, 167, 1321–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, C.; Zhou, L.; Chiou, K.; Huang, J. Multifunctional graphene hair dye. Chem 2018, 4, 784–794. [Google Scholar] [CrossRef] [Green Version]
- Battistella, C.; McCallum, N.C.; Gnanasekaran, K.; Zhou, X.; Caponetti, V.; Montalti, M.; Gianneschi, N.C. Mimicking natural human hair pigmentation with synthetic melanin. ACS Cent. Sci. 2020, 6, 1179–1188. [Google Scholar] [CrossRef]
- Battistella, C.; McCallum, N.C.; Vanthournout, B.; Forman, C.J.; Ni, Q.Z.; La Clair, J.J.; Burkat, M.D.; Shawkey, M.D.; Gianneschi, N.C. Bioinspired chemoenzymatic route to artificial melanin for hair pigmentation. Chem. Mater. 2020, 32, 9201–9210. [Google Scholar] [CrossRef]
- Dong, Y.Y.; Qiu, Y.; Gao, D.; Zhang, K.L.; Zhou, K.; Yin, H.G.; Yi, G.Y.; Li, J.; Xia, Z.N.; Fu, Q.F. Melanin-mimetic multicolor and low-toxicity hair dye. RSC Adv. 2019, 9, 33617–33624. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.F.; Wang, X.Y.; Gao, J.B.; Xia, F. Rapid preparation of polydopamine coating as a multifunctional hair dye. RSC Adv. 2019, 9, 20492–20496. [Google Scholar] [CrossRef] [Green Version]
- Im, K.M.; Kim, T.W.; Jeon, J.R. Metal-chelation assisted deposition of polydopamine on human hair: A ready-to use eumelanin-based hair dyeing methodology. ACS Biomater. Sci. Eng. 2017, 3, 628–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Kim, W.I.; Youn, W.; Seo, J.; Kim, B.J.; Lee, J.K.; Choi, I.S. Enzymatic film formation of nature-derived phenolic amines. Nanoscale 2018, 10, 13351–13355. [Google Scholar] [CrossRef] [PubMed]
- Tiampasook, P.; Chaiyasut, C.; Sivamaruthi, B.S.; Timudom, T.; Nacapunchai, D. Effect of Phyllanthus emblica Linn. on tensile strength of virgin and bleached hairs. Appl. Sci. 2020, 10, 6305. [Google Scholar] [CrossRef]
- Singh, V.; Ali, M.; Upadhyay, S. Study of colouring effect of herbal hair formulations on graying hair. Pharmacogn. Res. 2015, 7, 259–262. [Google Scholar] [CrossRef] [Green Version]
- Sivaram, G.; Malini, S.; Babu, G. Review of Ayurvedic herbs with Kesharanjana property in the management of canities (Palitya). Int. J. Ayurvedic Med. 2018, 9, 9–12. [Google Scholar]
- Han, S.Y.; Hong, S.-P.; Kang, E.K.; Kim, B.J.; Lee, H.; Kim, W.I.; Choi, I.S. Iron gall ink revisited: Natural formulation for black hair-dyeing. Cosmetics 2019, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef]
- Bat-Yehouda, M.Z. Les Encres Noires au Moyen Age (jusqu’à 1600) [Black Inks in the Middle Ages (until 1600)]; Centre National de la Recherche Scientifique: Paris, France, 1983; pp. 96–97. [Google Scholar]
- Pone, A.; Brostoff, L.B.; Gibbons, S.K.; Zavalij, P.; Virgah, C.; Hooper, J.; Alnemart, S.; Gaskell, K.J.; Eichhorn, B. Elucidation of the Fe(III) gallate structure in historical iron gall ink. Anal. Chem. 2016, 88, 5152–5258. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kim, W.I.; Youn, W.; Park, T.; Lee, S.; Kim, T.-S.; Mano, J.F.; Choi, I.S. Iron gall ink revisited: In situ oxidation of Fe(II)-tannin complex for fluidic-interface engineering. Adv. Mater. 2018, 30, 1805091. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Lee, J.K.; Choi, I.S. Iron gall ink revisited: Hierarchical formation of Fe(III)-tannic acid coacervate particles in microdroplets for protein condensation. Chem. Commun. 2019, 55, 2142–2145. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, J.; Han, S.Y.; Han, S.; Youn, W.; Choi, H.; Yun, G.; Choi, I.S. Ascorbic acid-mediated reductive disassembly of Fe3+-tannic acid shells in degradable single-cell nanoencapsulation. Chem. Commun. 2020, 56, 13748–13751. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ping, Y.; Ejima, H.; Alt, K.; Meissner, M.; Richardson, J.J.; Yan, Y.; Peter, K.; von Elverfeldt, D.; Hagemeyer, C.E.; et al. Engineering multifunctional capsules through the assembly of metal-phenolic networks. Angew. Chem. Int. Ed. 2014, 53, 5546–5551. [Google Scholar] [CrossRef]
- Battino, R.; Rettich, T.R.; Tominaga, T. The solubility of oxygen and ozone in liquids. J. Phys. Chem. Ref. Data 1983, 12, 163–178. [Google Scholar] [CrossRef]
- Durmus, D. CIELAB color space boundaries under theoretical spectra and 99 test color samples. Color Res. Appl. 2020, 45, 796–802. [Google Scholar] [CrossRef]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, S.; Moon, H.C.; Seo, H.; Kim, J.Y.; Hong, S.-P.; Lee, B.S.; Kang, E.; Lee, J.; Ryu, D.H.; et al. Antimicrobial spray nanocoating of supramolecular Fe(III)-tannic acid metal-organic coordination complex: Applications to shoe insoles and fruits. Sci. Rep. 2017, 7, 6980. [Google Scholar] [CrossRef]
- Dias, M.F.R.G.; de Almeida, A.M.; Cecato, P.M.R.; Adriano, A.R.; Pichler, J. The Shampoo pH can affect the hair: Myth or reality? Int. J. Trichology 2014, 6, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.Y.; Park, M.A.; Kim, Y.C. Peppermint oil promotes hair growth without toxic signs. Toxicol. Res. 2014, 30, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Panahi, Y.; Taghizadeh, M.; Marzony, E.T.; Sahebkar, A. Rosemary oil vs Minoxidil 2% for the treatment of androgenetic alopecia: A randomized comparative trial. Skinmed 2015, 13, 15–21. [Google Scholar] [PubMed]
- Abd, E.; Benson, H.A.E.; Roberts, M.S.; Grice, J.E. Minoxidil skin delivery from nanoemulsion formulations containing eucalyptol or oleic acid: Enhanced diffusivity and follicular targeting. Pharmaceutics 2018, 10, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Sample | L*sample | L*measured after Dyeing | L*measured after Shampooing | %ΔL* after Dyeing | %ΔL* after Shampooing |
|---|---|---|---|---|---|
| AO | 77.3 ± 1.5 | 25.6 ± 2.5 | 28.2 ± 2.3 | 66.9 | 63.5 |
| OO | 77.8 ± 1.6 | 22.5 ± 1.7 | 23.9 ± 1.9 | 71.1 | 69.3 |
| SFO | 77.9 ± 1.8 | 23.7 ± 1.8 | 26.6 ± 1.9 | 69.6 | 65.9 |
| GSO | 77.0 ± 1.3 | 22.4 ± 1.5 | 24.9 ± 1.7 | 70.9 | 67.7 |
| HSO | 78.6 ± 1.3 | 23.1 ± 3.1 | 24.7 ± 1.8 | 70.6 | 68.6 |
| PO | 78.6 ± 1.3 | 24.2 ± 1.7 | 25.8 ± 2.0 | 69.2 | 67.2 |
| RO | 77.8 ± 1.8 | 25.1 ± 1.6 | 27.8 ± 1.6 | 67.7 | 64.3 |
| Y-YO | 78.4 ± 1.3 | 21.3 ± 1.8 | 21.4 ± 1.8 | 72.8 | 72.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.Y.; Kang, E.K.; Choi, I.S. Iron Gall Ink Revisited: A Surfactant-Free Emulsion Technology for Black Hair-Dyeing Formulation. Cosmetics 2021, 8, 9. https://0-doi-org.brum.beds.ac.uk/10.3390/cosmetics8010009
Han SY, Kang EK, Choi IS. Iron Gall Ink Revisited: A Surfactant-Free Emulsion Technology for Black Hair-Dyeing Formulation. Cosmetics. 2021; 8(1):9. https://0-doi-org.brum.beds.ac.uk/10.3390/cosmetics8010009
Chicago/Turabian StyleHan, Sang Yeong, Eunhye K. Kang, and Insung S. Choi. 2021. "Iron Gall Ink Revisited: A Surfactant-Free Emulsion Technology for Black Hair-Dyeing Formulation" Cosmetics 8, no. 1: 9. https://0-doi-org.brum.beds.ac.uk/10.3390/cosmetics8010009