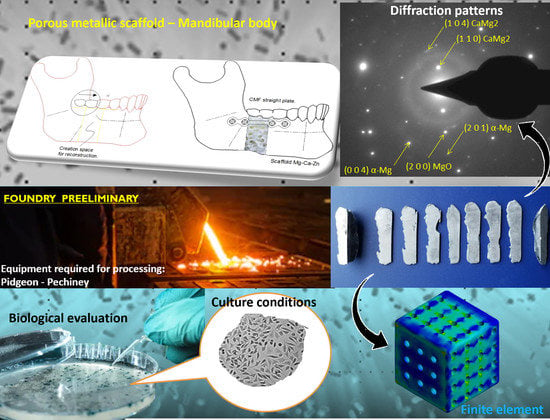
Bio-Fabrication and Experimental Validation of an Mg - 25Ca - 5Zn Alloy Proposed for a Porous Metallic Scaffold
Abstract
:1. Introduction
2. Materials
3. Methods
3.1. SEM-EDS
3.2. XRD
3.3. TEM-FIB
3.4. Micro-Indentation
3.5. Cell Viability
4. Results
4.1. SEM-EDS
4.2. XRD
4.3. TEM
4.4. Micro-Indentation
4.5. Cell Viability
4.6. Validation by Finite Element Method, Load Progression versus Comparison to BDM
5. Discussion
6. Conclusions
- (1)
- The optimization of the casting process of a Mg-Ca-Zn alloy has a microstructural limit, which is due to the eutectic mixture. Even when the process is optimized, a fine dispersion between phases will continue to appear, resulting in an origin for crack nucleation.
- (2)
- The period of exposure to elements such as Mg, Ca and Zn within the body causes pathophysiology to occur. Being clear about the period of exposure in days for the effects to occur is a key factor in understanding the beneficial effect that they can contribute prior to hydrolytic degradation in vivo.
- (3)
- Zn improves the mechanical response when hardening this type of alloys, however, exceeding the permitted limits damages the toxicological limit, which will have an impact on cell viability. Viz, Zhuang, et al. reported Zn dose-dependent and over-boosted concentration caused cell death due to cytotoxicity [90]. Elements such as Sr and Ca have been reported to be able to counteract and reduce the toxicity of Zn [133,134]. Lu et al. reported that Zn promotes the absorption of the hydrogen that is released due to the Mg reaction in vitro [135].
- (4)
- An annealing process on this alloy will dissolve the intermetallic phases that occur and help to homogenize the distribution of the solutes in the Mg matrix, however, the precipitates could have a detrimental effect on the corrosion rate.
- (5)
- The melting of Mg, Ca and Zn and their modification by diffusion are possible from MgCa master alloys. This process manages to diffuse Zn in powder over MgCa; the use of a vacuum system will allow a clean atmosphere. Thus, the alloy microstructure and grain size will be finer.
- (6)
- The coatings on Mg alloys are very important, depending on the type of medical application for which they are to be used. A coating used on Mg alloys has the purpose of increasing the implant time in vivo, which will provide mechanical resistance to the fracture; for this application, using a coating that reduces the appearance of pitting corrosion is a multidisciplinary strategy that will completely depend on the initial weeks, during which it is necessary to maintain the mechanical integrity of the implant. It is suggested to evaluate a coating based on Chitosan that allows the maintenance of an intact implant surface for six weeks to limit the places where crack propagation can begin.
- (7)
- The elastic modulus was 74.2 GPa for the longitudinal direction and 66.2 GPa in the transverse direction. This was determined from the repetitions of the test, which showed that the elastic modulus was always higher in the longitudinal direction, while the hardness followed a similar pattern, reported as 278.9 and 264.3 HV in longitudinal and transverse direction, respectively. An anisotropy condition between the longitudinal and transverse direction is fulfilled by having different elastic modulus and hardness. The determination of the elastic modulus and hardness in each triad for each degree of freedom could be useful to relate to the orientation of the planes and to have the behavior of the elastic constants characterized.
- (8)
- The ideal application for this type of alloy corresponds to anatomical sites where it is only exposed to gravity and forces caused by muscles, such as the movement of the upper extremities and the skull.
- (9)
- Experimenting with texture control techniques on Mg alloys could be an effective strategy to determine the ideal method for manufacturing Mg-based implants. However, the mechanical behavior of Mg depends on the adequate relationship between the volumetric fraction of elements and the formation of lamellar long period stacking order (LPSO) secondary phases [127,136,137].
7. Declarations
- Ethics approval and consent to participate: N/A
- Consent for publication: N/A
- Availability of data and materials: OK
- Competing interests: No conflict of interest.
- Funding
- N/A
- Authors’ contributions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Holtzclaw, D.; Toscano, N.; Eisenlohr, L.; Callan, D. The safety of bone allografts used in dentistry: A review. J. Am. Dent. Assoc. 2008, 139, 1192–1199. [Google Scholar] [CrossRef]
- Salyer, K.E.; Taylor, D.P. Bone grafts in craniofacial surgery. Clin. Plast. Surg. 1987, 14, 27–35. [Google Scholar] [CrossRef]
- Wang, J.C.; Alanay, A.; Mark, D. A comparison of commercially available demineralized bone matrix for spinal fusion. Eur. Spine J. 2007, 16, 1233–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, H.J.; Julmi, S. Magnesium Alloys for Open-Pored Bioresorbable Implants. JOM 2020, 72, 1859–1869. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Ren, Z.; Xu, Y.; Pang, S.; Zhao, X.; Zhao, Y. Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Scanning 2018, 2018, 19. [Google Scholar] [CrossRef] [Green Version]
- Ramirez Patino, J.F. Bioresorbable Magnesium-Based Sponge and Foam Materials. Methods and Devices. Worldwide Applications WO 2018/187756 A1, 11 October 2018. [Google Scholar]
- Becerra, L.H.C.; Rodríguez, M.A.L.H.; Solís, H.E.; Arroyo, R.L.; Castro, A.T. Bio-inspired biomaterial Mg-Zn-Ca: A review of the main mechanical and biological properties of Mg-based alloys. Biomed. Phys. Eng. Express 2020, 6, 042001. [Google Scholar] [CrossRef]
- Li, X.; Niu, Y.; Guo, H.; Chen, H.; Li, F.; Zhang, J.; Chen, W.; Wu, Z.; Deng, Y.; Wei, J.; et al. Preparation and osteogenic properties of magnesium calcium phosphate biocement scaffolds for bone regeneration. J. Instrum. 2013, 8, C07010. [Google Scholar] [CrossRef]
- Deng, J.; Ye, J.; Zhao, Y.; Zhu, Y.; Wu, T.; Zhang, C.; Dong, L.; Ouyang, H.; Cheng, X.; Wang, X. ZnO and Hydroxyapatite-Modified Magnesium Implant with a Broad Spectrum of Antibacterial Properties and a Unique Minimally Invasive Defined Degrading Capability. ACS Biomater. Sci. Eng. 2019, 5, 4285–4292. [Google Scholar] [CrossRef]
- Alshaaer, M.; Abdel-Fattah, E.; Saadeddin, I.; al Battah, F.; Issa, K.; Saffarini, G. The effect of natural fibres template on the chemical and structural properties of Biphasic Calcium Phosphate scaffold. Mater. Res. Express 2020, 7, 065405. [Google Scholar] [CrossRef]
- Moradi, E.; Ebrahimian-Hosseinabadi, M.; Khodaei, M.; Toghyani, S. Magnesium/Nano-Hydroxyapatite porous biodegradable composite for biomedical applications. Mater. Res. Express 2019, 6, 075408. [Google Scholar] [CrossRef]
- Ong, K.L.; Yun, B.M.; White, J.B. New biomaterials for orthopedic implants. Orthop. Res. Rev. 2015, 7, 107–130. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food & Drug Administration. Jurisdictional Update: Human Demineralized Bone Matrix. 2018. Available online: https://www.fda.gov/combination-products/jurisdictional-updates/jurisdictional-update-human-demineralized-bone-matrix (accessed on 12 October 2021).
- Wang, J.L.; Xu, J.K.; Hopkins, C.; Chow, D.H.K.; Qin, L. Biodegradable Magnesium-Based Implants in Orthopedics—A General Review and Perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [PubMed]
- Amerstorfer, F.; Fischerauer, S.F.; Fischer, L.; Eichler, J.; Draxler, J.; Zitek, A.; Meischel, M.; Martinelli, E.; Kraus, T.; Hann, S.; et al. Long-term in vivo degradation behavior and near-implant distribution of resorbed elements for magnesium alloys WZ21 and ZX50. Acta Biomater. 2016, 42, 440–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesz, S.; Kraczla, J.; Nowosielski, R. Structure and compression strength characteristics of the sintered Mg–Zn–Ca–Gd alloy for medical applications. Arch. Civ. Mech. Eng. 2018, 18, 1288–1299. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Abdellahi, M.; Hamzah, E.; Ismail, A.F.; Bahmanpour, M. Modelling corrosion rate of biodegradable magnesium-based alloys: The case study of Mg-Zn-RE-xCa (x = 0, 0.5, 1.5, 3 and 6 wt%) alloys. J. Alloys Compd. 2016, 687, 630–642. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.; Wei, Y.; Qiao, Y. Effects of magnesium-calcium alloys with different calcium content on their mechanical properties. IOP Conf. Ser. Mater. Sci. Eng. 2020, 735, 012010. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloy. 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Pulido-gonzález, N.; Torres, B.; Rodrigo, P.; Hort, N.; Rams, J. Microstructural, mechanical and corrosion characterization of an as-cast Mg–3Zn–0.4Ca alloy for biomedical applications. J. Magnes. Alloy. 2020, 8, 510–522. [Google Scholar] [CrossRef]
- Radha, R.; Sreekanth, D. Insight of magnesium alloys and composites for orthopedic implant applications—A review. J. Magnes. Alloy. 2017, 5, 286–312. [Google Scholar] [CrossRef]
- Xu, T.O.; Kim, H.S.; Stahl, T.; Nukavarapu, S.P. Self-neutralizing PLGA/magnesium composites as novel biomaterials for tissue engineering. Biomed. Mater. 2018, 13, 035013. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.N.; Li, N.; Zhou, W.R.; Zheng, Y.F.; Zhao, X.; Cai, Q.Z.; Ruan, L. Corrosion resistance and surface biocompatibility of a microarc oxidation coating on a Mg-Ca alloy. Acta Biomater. 2011, 7, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Sedelnikova, M.B.; Komarova, E.G.; Sharkeev, Y.P.; Tolkacheva, T.V.; Sheikin, V.V.; Egorkin, V.S.; Mashtalyar, D.V.; Kazakbaeva, A.A.; Schmidt, J. Characterization of the Micro-Arc coatings containing β-tricalcium phosphate particles on Mg-0.8Ca alloy. Metals 2018, 8, 238. [Google Scholar] [CrossRef] [Green Version]
- Farahany, S.; Bakhsheshi-Rad, H.R.; Idris, M.H.; Kadir, M.R.A.; Lotfabadi, A.F.; Ourdjini, A. In-situ thermal analysis and macroscopical characterization of Mg-xCa and Mg-0.5Ca-xZn alloy systems. Thermochim. Acta 2012, 527, 180–189. [Google Scholar] [CrossRef]
- Zeng, R.; Lan, Z.; Kong, L.; Huang, Y.; Cui, H. Characterization of calcium-modified zinc phosphate conversion coatings and their influences on corrosion resistance of AZ31 alloy. Surf. Coat. Technol. 2011, 205, 3347–3355. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.Z.Q.; Wang, C.; Yang, J.; Guo, C. Mineralized collagen/Mg-Ca alloy combined scaffolds with improved biocompatibility for enhanced bone response following tooth extraction. Biomed. Mater. 2018, 13, 065008. [Google Scholar] [CrossRef]
- Feyerabend, R.W.F.; Witte, F.; Vogt, C.; Fischer, J.; Schreyer, A.; Kainer, K.U.; Hort, N. In Vitro Testing of Magnesium Alloys—Challenges and Options. In Proceedings of the 8th International Conference on Magnesium Alloys and their Applications, Weimar, Germany, 26–29 October 2021. [Google Scholar]
- Sharma, P.; Chattopadhyaya, S.; Singh, N.K. A review on magnetically supported gas metal arc welding process for magnesium alloys. Mater. Res. Express 2019, 6, 082002. [Google Scholar] [CrossRef]
- Toghyani, S.; Khodaei, M. Fabrication and characterization of magnesium scaffold using different processing parameters. Mater. Res. Express 2018, 5, 035407. [Google Scholar] [CrossRef]
- Guan, R.g.; Cipriano, A.F.; Zhao, Z.; Lock, J.; Tie, D.; Zhao, T.; Cui, T.; Liu, H. Development and evaluation of a magnesium-zinc-strontium alloy for biomedical applications—Alloy processing, microstructure, mechanical properties, and biodegradation. Mater. Sci. Eng. C 2013, 33, 3661–3669. [Google Scholar] [CrossRef]
- Annur, D.; Franciska, P.; Erryani, A.; Amal, M.I.; Sitorus, L.S.; Kartika, I. The synthesis and characterization of Mg-Zn-Ca alloy by powder metallurgy process. AIP Conf. Proc. 2016, 1725, 19. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Narayan, R.J. Structural and optical properties of hexagonal Mg xZn 1-xO thin films. J. Electron. Mater. 2006, 35, 869–876. [Google Scholar] [CrossRef]
- Mironov, V.; Trusk, T.; Kasyanov, V.; Little, S.; Swaja, R.; Markwald, R. Biofabrication: A 21st century manufacturing paradigm. Biofabrication 2009, 1, 022001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, M.; Niu, W.; Wong, D.W.C.; Zeng, W.; Mei, J.; Zhang, M. Finite element analysis of locking plate and two types of intramedullary nails for treating mid-shaft clavicle fractures. Injury 2016, 47, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Senkov, O.N.; Scott, J.M. Glass forming ability and thermal stability of ternary Ca-Mg-Zn bulk metallic glasses. J. Non. Cryst. Solids 2005, 351, 3087–3094. [Google Scholar] [CrossRef]
- Gu, X.; Zheng, Y.; Zhong, S.; Xi, T.; Wang, J.; Wang, W. Corrosion of, and cellular responses to Mg-Zn-Ca bulk metallic glasses. Biomaterials 2010, 31, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Geanta, V.; Voiculescu, I.; Kelemen, H.; Manu, D.; Molnár, G.; Kelemen, G. Mg-Ca-Zn bio-degradable light alloys produced in a levitation induction melting furnace. Int. J. Appl. Electromagn. Mech. 2020, 63, S69–S78. [Google Scholar] [CrossRef]
- Hrapkowicz, B.; Lesz, S.T. Characterization of Ca 50 Mg 20 Zn 12 Cu 18 Alloy. Arch. Foundry Eng. 2019, 19, 75–82. [Google Scholar] [CrossRef]
- Ma, S.; Xing, F.; Ta, N.; Zhang, L. Kinetic modeling of high-temperature oxidation of pure Mg. J. Magnes. Alloy. 2020, 8, 819–831. [Google Scholar] [CrossRef]
- Shahri, Z.; Allahkaram, S.R.; Soltani, R.; Jafari, H. Optimization of plasma electrolyte oxidation process parameters for corrosion resistance of Mg alloy. J. Magnes. Alloy. 2020, 8, 431–440. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, P.M. Development of Mg based biomaterial with improved mechanical and degradation properties using powder metallurgy. J. Magnes. Alloy. 2020, 8, 883–898. [Google Scholar] [CrossRef]
- Yang, M.; Liu, D.; Zhang, R.; Chen, M. Microstructure and Properties of Mg-3Zn-0.2Ca Alloy for Biomedical Application. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Met. Mater. Eng. 2018, 47, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.E. Aggie Digital Collections and Scholarship Processing and Characterization Of Innovative Magnesium Alloys for Biodegradable Orthopaedic Implants. Ph.D. Thesis, 2014. Available online: https://digital.library.ncat.edu/dissertations/102 (accessed on 12 October 2021).
- Bell, S.; Ajami, E.; Davies, J.E. An improved mechanical testing method to assess bone-implant anchorage. J. Vis. Exp. 2014, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischerauer, S.F. Preclinical Characterization of Bioresorbable Magnesium Implants for Osteosynthesis. Ph.D. Thesis, Medical University of Graz, Graz, Austria, 2015. [Google Scholar]
- Liu, C.; Wang, J.; Gao, C.; Wang, Z.; Zhou, X.; Tang, M.; Yu, K.; Deng, Y. Enhanced osteoinductivity and corrosion resistance of dopamine/gelatin/rhBMP-2–coated β-TCP/Mg-Zn orthopedic implants: An in vitro and in vivo study. PLoS ONE 2020, 15, e0228247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiromoto, S.; Itoh, S.; Noda, N.; Yamazaki, T.; Katayama, H.; Akashi, T. Osteoclast and osteoblast responsive carbonate apatite coatings for biodegradable magnesium alloys. Sci. Technol. Adv. Mater. 2020, 21, 346–358. [Google Scholar] [CrossRef]
- Chang, Y.H.; Tseng, C.C.; Chao, C.Y.; Chen, C.H.; Lin, S.Y.; Du, J.K. Mg-Zn-Ca alloys for hemostasis clips for vessel ligation: In vitro and in vivo studies of their degradation and response. Materials 2020, 13, 3039. [Google Scholar] [CrossRef]
- Sarkar, K.; Kumar, V.; Devi, K.B.; Ghosh, D.; Nandi, S.K.; Roy, M. Anomalous in Vitro and in Vivo Degradation of Magnesium Phosphate Bioceramics: Role of Zinc Addition. ACS Biomater. Sci. Eng. 2019, 5, 5097–5106. [Google Scholar] [CrossRef]
- Ostrowski, N.; Lee, B.; Hong, D.; Enick, P.N.; Roy, A.; Kumta, P.N. Synthesis, Osteoblast, and Osteoclast Viability of Amorphous and Crystalline Tri-Magnesium Phosphate. ACS Biomater. Sci. Eng. 2015, 1, 52–63. [Google Scholar] [CrossRef]
- Ibrahim, H.; Esfahani, S.N.; Poorganji, B.; Dean, D.; Elahinia, M. Resorbable bone fixation alloys, forming, and post-fabrication treatments. Mater. Sci. Eng. C 2017, 70, 870–888. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.H.; Guan, S.K.; Chen, J.; Wang, L.G.; Zhu, S.J.; Hu, J.H.; Ren, Z.W. Fabrication and characterization of rod-like nano-hydroxyapatite on MAO coating supported on Mg-Zn-Ca alloy. Appl. Surf. Sci. 2011, 257, 2231–2237. [Google Scholar] [CrossRef]
- Xin, Y.; Hu, T.; Chu, P.K. Influence of Test Solutions on In Vitro Studies of Biomedical Magnesium Alloys. J. Electrochem. Soc. 2010, 157, C238–C243. [Google Scholar] [CrossRef]
- Yan, Y.; Chu, X.; Luo, X.; Xu, X.; Zhang, Y.; Dai, Y.; Li, D.; Chen, L.; Xiao, T.; Yu, K. A homogenous microstructural Mg-based matrix model for orthopedic application with generating uniform and smooth corrosion product layer in Ringer’s solution: Study on biodegradable behavior of Mg-Zn alloys prepared by powder metallurgy as a case. J. Magnes. Alloy. 2021, 9, 225–240. [Google Scholar] [CrossRef]
- Vinogradov, A.; Vasilev, E.; Kopylov, V.I.; Linderov, M.; Brilevesky, A.; Merson, D. High performance fine-grained biodegradable Mg-Zn-Ca alloys processed by severe plastic deformation. Metals 2019, 9, 186. [Google Scholar] [CrossRef] [Green Version]
- Priyanto, T.H.; Insani, A.; Muslih, R.; Bharoto. Texture Analysis on the AZ31 Magnesium Alloy Using Neutron Diffraction Method. J. Phys. Conf. Ser. 2020, 1436, 235. [Google Scholar] [CrossRef]
- Park, M.; Kim, K. Basal texture formation behavior of M1 magnesium alloy during high-temperature compression deformation. J. Phys. Conf. Ser. 2019, 1270. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; You, Z.; Liu, S.; Guan, K.; Wu, R.; Wang, J.; Feng, J. Towards developing Mg alloys with simultaneously improved strength and corrosion resistance via RE alloying. J. Magnes. Alloy. 2021, 9, 41–56. [Google Scholar] [CrossRef]
- Du, P.; Furusawa, S.; Furushima, T. Microstructure and performance of biodegradable magnesium alloy tubes fabricated by local-heating-assisted dieless drawing. J. Magnes. Alloy. 2020, 8, 614–623. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Chen, H.T.; Zhang, K.; Dai, F.Z.; Liu, X.F. Crystallography of precipitates in Mg alloys. J. Magnes. Alloy. 2021, 9, 416–431. [Google Scholar] [CrossRef]
- Afandi, R.; Sutiyoko; Lutiyatm. Characteristic modifications of magnesium and its alloy for future implant material—Review. J. Phys. Conf. Ser. 2020, 1517. [Google Scholar] [CrossRef]
- Janeček, M.; Krajňák, T.; Minárik, P.; Čížek, J.; Stráská, J.; Stráský, J. Structural stability of ultra-fine grained magnesium alloys processed by equal channel angular pressing. IOP Conf. Ser. Mater. Sci. Eng. 2017, 194, 12022. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Xu, G.; Liu, D.; Zhao, Y.; Ning, B.; Chen, M. Study on the Microstructure, Mechanical Properties and Corrosion Behavior of Mg-Zn-Ca Alloy Wire for Biomaterial Application. J. Mater. Eng. Perform. 2018, 27, 1837–1846. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Yoo, H.-S.; Son, H.-T. Microstructure and Mechanical Properties of Mg–11Li–6Zn–0.6Zr–0.4Ag–0.2Ca–x Y Alloys. J. Nanosci. Nanotechnol. 2018, 18, 6304–6308. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Hamidi, M.F.F.A.; Harun, W.S.W.; Samykano, M.; Ghani, S.A.C.; Ghazalli, Z.; Ahmad, F.; Sulong, A.B. A review of biocompatible metal injection moulding process parameters for biomedical applications. Mater. Sci. Eng. C 2018, 78, 1263–1276. [Google Scholar] [CrossRef] [Green Version]
- Vlček, M.; Lukáč, F.; Kudrnová, H.; Smola, B.; Stulíková, I.; Luczak, M.; Szakács, G.; Hort, N.; Willumeit-Römer, R. Microhardness and in vitro corrosion of heat-treated Mg-Y-Ag biodegradable alloy. Materials 2017, 10, 55. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg-Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Cha, P.R.; Han, H.S.; Yang, G.F.; Kim, Y.C.; Hong, K.H.; Lee, S.C.; Jung, J.Y.; Ahn, J.P.; Kim, Y.Y.; Cho, S.Y.; et al. Biodegradability engineering of biodegradable Mg alloys: Tailoring the electrochemical properties and microstructure of constituent phases. Sci. Rep. 2013, 3, 2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.C.; Li, M.H.; Hu, W.Y.; Hodgson, P.D.; Wen, C.E. Biodegradable Mg-Ca and Mg-Ca-Y Alloys for Regenerative Medicine. Mater. Sci. Forum 2010, 654–656, 2192–2195. [Google Scholar] [CrossRef]
- Li, T.; He, Y.; Zhang, H.; Wang, X. Microstructure, mechanical property and in vitro biocorrosion behavior of single-phase biodegradable Mg-1.5Zn-0.6Zr alloy. J. Magnes. Alloy 2014, 2, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants—A Review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Bradshaw, A.R.; Chiu, Y.L.; Jones, I.P. Effects of secondary phase and grain size on the corrosion of biodegradable Mg-Zn-Ca alloys. Mater. Sci. Eng. C 2015, 48, 480–486. [Google Scholar] [CrossRef]
- Sikora-Jasinska, M.; Mostaed, E.; Mostaed, A.; Beanland, R.; Mantovani, D.; Vedani, M. Fabrication, mechanical properties and in vitro degradation behavior of newly developed Zn Ag alloys for degradable implant applications. Mater. Sci. Eng. C 2017, 77, 1170–1181. [Google Scholar] [CrossRef]
- Jia, H.M.; Feng, X.H.; Yang, Y.S. Microstructure of directionally solidified Mg-Zn alloy with different growth rates. Mater. Sci. Forum 2015, 816, 411–417. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Fereidouni-Lotfabadi, A.; Daroonparvar, M.; Yajid, M.A.M.; Mezbahul-Islam, M.; Kasiri-Asgarani, M.; Medraj, M. Microstructure and bio-corrosion behavior of Mg-Zn and Mg-Zn-Ca alloys for biomedical applications. Mater. Corros. 2014, 65, 1178–1187. [Google Scholar] [CrossRef]
- Zivić, F.; Grujović, N.; Manivasagam, G.; Richard, C.; Landoulsi, J.; Petrović, V. The potential of magnesium alloys as bioabsorbable/biodegradable implants for biomedical applications. Tribol. Ind. 2014, 36, 67–73. [Google Scholar]
- Tao, J.X.; Zhao, M.-C.; Zhao, Y.-C.; Yin, D.-F.; Liu, L.; Gao, C.; Shuai, C.; Atrens, A. Influence of graphene oxide (GO) on microstructure and biodegradation of ZK30-xGO composites prepared by selective laser melting. J. Magnes. Alloy. 2020, 8, 952–962. [Google Scholar] [CrossRef]
- Medina, S.; Mendoza, E.; Meza, J.M. Comportamiento microestructural de una fundición de magnesio puro con control en la temperatura de solidificación. Univ. Tecnológica Pereira, Scientia Tech. Año XXII 2017, 22, 145–149. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Dou, X.; Wang, J.; Huang, Y.; Gavras, S.; Hort, N.; Liu, S.; Hu, H.; Wang, J.; Pan, F. Achieving enhanced mechanical properties in Mg-Gd-Y-Zn-Mn alloy by altering dynamic recrystallization behavior via pre-ageing treatment. Mater. Sci. Eng. A 2020, 790, 139635. [Google Scholar] [CrossRef]
- Jardim, P.M.; Solórzano, G.; Sande, J.B.V. Second phase formation in melt-spun Mg-Ca-Zn alloys. Mater. Sci. Eng. A 2004, 381, 196–205. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, S.; Roy, M. Recent Developments in Magnesium Metal-Matrix Composites for Biomedical Applications: A Review. ACS Biomater. Sci. Eng. 2020, 6, 4748–4773. [Google Scholar] [CrossRef]
- Bazhenov, V.; Koltygin, A.; Komissarov, A.; Li, A.; Bautin, V.; Khasenova, R.; Anishchenko, A.; Seferyan, A.; Komissarova, J.; Estrin, Y. Gallium-containing magnesium alloy for potential use as temporary implants in osteosynthesis. J. Magnes. Alloy. 2020, 8, 352–363. [Google Scholar] [CrossRef]
- Bowen, P.K.; McNamara, C.T.; Mills, O.P.; Drelich, J.; Goldman, J. FIB-TEM Study of Magnesium Corrosion Products after 14 Days in the Murine Artery. ACS Biomater. Sci. Eng. 2015, 1, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Moritz, N.; Strandberg, N.; Zhao, D.S.; Mattila, R.; Paracchini, L.; Vallittu, P.K.; Aro, H.T. Mechanical properties and in vivo performance of load-bearing fiber-reinforced composite intramedullary nails with improved torsional strength. J. Mech. Behav. Biomed. Mater. 2014, 40, 127–139. [Google Scholar] [CrossRef] [PubMed]
- ISO. ISO 10993-12:2012(en). In Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- Gai, X.; Liu, C.; Wang, G.; Qin, Y.; Fan, C.; Liu, J.; Shi, Y. A novel method for evaluating the dynamic biocompatibility of degradable biomaterials based on real-time cell analysis. Regen. Biomater. 2020, 7, 321–329. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, Q.; Jia, G.; Li, H.; Yuan, G.; Yu, H. A Biomimetic Zinc Alloy Scaffold Coated with Brushite for Enhanced Cranial Bone Regeneration. ACS Biomater. Sci. Eng. 2020. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.G.; Johnson, I.; Cui, T.; Zhao, T.; Zhao, Z.Y.; Li, X.; Liu, H. Electrodeposition of hydroxyapatite coating on Mg-4.0Zn-1.0Ca-0.6Zr alloy and in vitro evaluation of degradation, hemolysis, and cytotoxicity. J. Biomed. Mater. Res.—Part A 2012, 100A, 999–1015. [Google Scholar] [CrossRef] [PubMed]
- Cahyono, N.A.; Sulistyani, L.D.; Santosa, A.S.; Latief, B.S. Analysis of the mechanical properties of magnesium equal-channel angular pressing of a plate on mandibular fracture through bending and ductility tests in physiological fluids of Dulbecco’s Modified Eagle Medium. J. Phys. Conf. Ser. 2018, 1073, 062008. [Google Scholar] [CrossRef] [Green Version]
- Mayama, T.; Tane, M.; Tadano, Y. Crystal plasticity analysis of anisotropic deformation behavior of porous magnesium with oriented pores. J. Phys. Conf. Ser. 2018, 1063, 012047. [Google Scholar] [CrossRef] [Green Version]
- Fattah-alhosseini, A.; Chaharmahali, R.; Babaei, K. Effect of particles addition to solution of plasma electrolytic oxidation (PEO) on the properties of PEO coatings formed on magnesium and its alloys: A review. J. Magnes. Alloy. 2020, 8, 799–818. [Google Scholar] [CrossRef]
- Mguni, L.L.; Mukenga, M.; Muzenda, E.; Jalama, K.; Meijboom, R. Expanding the synthesis of Stöber spheres: Towards the synthesis of nano-magnesium oxide and nano-zinc oxide. J. Sol-Gel Sci. Technol. 2013, 66, 91–99. [Google Scholar] [CrossRef]
- De, A.K.; Mukhopadhyay, A.; Sen, S.; Puri, I.K. Numerical simulation of early stages of oxide formation in molten aluminium-magnesium alloys in a reverberatory furnace. Model. Simul. Mater. Sci. Eng. 2004, 12, 389–405. [Google Scholar] [CrossRef]
- Gulbransen, E.A. The Oxidation and Evaporation of Magnesium at Temperatures from 400° to 500 °C. Trans. Electrochem. Soc. 1945, 87, 589–599. [Google Scholar] [CrossRef]
- Ren, F.; Xin, R.; Ge, X.; Leng, Y. Characterization and structural analysis of zinc-substituted hydroxyapatites. Acta Biomater. 2009, 5, 3141–3149. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z.; LeGeros, J.P. Dense hydroxyapatite. An introduction to bioceramics. World Sci. 1993, 1. [Google Scholar]
- Tripathy, A.P.N.K.; Patel, P.N. Preparation, IR, and lattice constant measurements of mixed (Ca+Cu+Zn) hydroxylapatites. J. Solid State Chem. 1989, 80, 1–5. [Google Scholar] [CrossRef]
- Paul, S.; Ramasamy, P.; Das, M.; Mandal, D.; Renk, O.; Calin, M.; Eckert, J.; Bera, S. New Mg-Ca-Zn amorphous alloys: Biocompatibility, wettability and mechanical properties. Materialia 2020, 12, 100799. [Google Scholar] [CrossRef]
- Fazel Anvari-Yazdi, A.; Tahermanesh, K.; Hadavi, S.M.; Talaei-Khozani, T.; Razmkhah, M.; Abed, S.M.; Mohtasebi, M.S. Cytotoxicity assessment of adipose-derived mesenchymal stem cells on synthesized biodegradable Mg-Zn-Ca alloys. Mater. Sci. Eng. C 2016, 69, 584–597. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, J.; Nguyen, N.T.; Guo, Y.; Xu, C.; Seo, C.; Villafana, E.; Jimenez, H.; Chai, Y.; Guan, R. Antimicrobial Bioresorbable Mg-Zn-Ca Alloy for Bone Repair in a Comparison Study with Mg-Zn-Sr Alloy and Pure Mg. ACS Biomater. Sci. Eng. 2020, 6, 517–538. [Google Scholar] [CrossRef]
- Rahman, M.; Li, Y.; Wen, C. HA coating on Mg alloys for biomedical applications: A review. J. Magnes. Alloy. 2020, 8, 929–943. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, C.; Wen, J.; Li, X.; Fu, L. Synthesis and structural characteristics of magnesium and zinc doped hydroxyapatite whiskers. Ceram.—Silikaty 2017, 61, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Rahim, M.I.; Babbar, A.; Lienenklaus, S.; Pils, M.C.; Rohde, M. Degradable magnesium implant-associated infections by bacterial biofilms induce robust localized and systemic inflammatory reactions in a mouse model. Biomed. Mater. 2017, 12, 055006. [Google Scholar] [CrossRef] [Green Version]
- Willumeit-Römer, R. The Interface Between Degradable Mg and Tissue. JOM 2019, 71, 1447–1455. [Google Scholar] [CrossRef] [Green Version]
- Cicha, I.; Singh, R.; Garlichs, C.D.; Alexiou, C. Nano-biomaterials for cardiovascular applications: Clinical perspective. J. Control. Release 2016, 229, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Banerjee, S.S.; Bandyopadhyay, A.; Bose, S. Zn- and Mg-doped hydroxyapatite nanoparticles for controlled release of protein. Langmuir 2010, 26, 4958–4964. [Google Scholar] [CrossRef] [Green Version]
- Nassif, N.; Ghayad, I. Corrosion protection and surface treatment of magnesium alloys used for orthopedic applications. Adv. Mater. Sci. Eng. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Rueda, L.M.; Nieves, C.; Barrios, C.A.H.; Coy, A.E.; Viejo, F. Design of TEOS-GPTMS sol-gel coatings on rare-earth magnesium alloys employed in the manufacture of orthopaedic implants. J. Phys. Conf. Ser. 2016, 687, 012013. [Google Scholar] [CrossRef]
- Duan, X.W.; Liu, J.J.; Liu, L.L.; Gong, B.; Li, P.; Liu, B.S. Study on Constitutive Model and Dynamic Recrystallization Softening Behavior of AZ80A Magnesium Alloy. Mater. Res. Express. 2017, 6, 126576. [Google Scholar] [CrossRef]
- Gummow, R.J.; He, Y. Morphology and Preferred Orientation of Pulse Electrodeposited Magnesium. J. Electrochem. Soc. 2010, 157, E45–E49. [Google Scholar] [CrossRef]
- Yan, J.; Qin, Z.; Yan, K. Mechanical properties and microstructure evolution of Mg-6 wt% Zn alloy during equal-channel angular pressing. Metals 2018, 8, 841. [Google Scholar] [CrossRef] [Green Version]
- Tong, X.; Wu, G.; Zhang, L.; Wang, Y.; Liu, W.; Ding, W. Microstructure and mechanical properties of repair welds of low-pressure sand-cast Mg–Y–RE–Zr alloy by tungsten inert gas welding. J. Magnes. Alloy. 2020. [Google Scholar] [CrossRef]
- Yao, X.; Tang, J.; Zhou, Y.; Atrens, A.; Dargusch, M.S.; Wiese, B.; Ebel, T.; Yan, M. Surface modification of biomedical Mg-Ca and Mg-Zn-Ca alloys using selective laser melting: Corrosion behaviour, microhardness and biocompatibility. J. Magnes. Alloy. 2020. [Google Scholar] [CrossRef]
- Chen, J.; Feng, J.; Yan, L.; Li, H.; Xiong, C.; Ma, S. In situ growth process of Mg–Fe layered double hydroxide conversion film on MgCa alloy. J. Magnes. Alloy. 2020. [Google Scholar] [CrossRef]
- Meibom, A.; Cuif, J.-P.; Hillion, F.; Constantz, B.R.; Juillet-Leclerc, A.; Dauphin, Y.; Watanabe, T.; Dunbar, R.B. Distribution of magnesium in coral skeleton. Geophys. Res. Lett. 2017, 31. [Google Scholar] [CrossRef]
- Krishnan, K. Effect of microstructures and textures on the anisotropy of mechanical properties of AZ31 magnesium alloy sheets subjected to high strain rate rolling. J. Phys. D Appl. Phys. 2019, in press. [Google Scholar]
- Lee, J.; Lee, Y.-S.; Lee, M.-G.; Kim, D. Constitutive modelling of magnesium alloy sheets under strain path changes. J. Phys. Conf. Ser. 2016, 734, 032135. [Google Scholar] [CrossRef]
- Sułkowski, B.; Janoska, M.; Boczkal, G.; Chulist, R.; Mroczkowski, M.; Pałka, P. The effect of severe plastic deformation on the Mg properties after CEC deformation. J. Magnes. Alloy. 2020, 8, 761–768. [Google Scholar] [CrossRef]
- Atrens, A.; Liu, M.; Abidin, N.I.Z. Corrosion mechanism applicable to biodegradable magnesium implants. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2011, 176, 1609–1636. [Google Scholar] [CrossRef]
- An, L.; Ma, Y.; Sun, L.; Wang, Z.; Wang, S. Investigation of mutual effects among additives in electrolyte for plasma electrolytic oxidation on magnesium alloys. J. Magnes. Alloy. 2020, 8, 523–536. [Google Scholar] [CrossRef]
- Cesarz-Andraczke, K.; Nowosielski, R. Surface Structure and Corrosion Behavior of Mg 68−x Zn 28+x Ca 4 (x = 0.4) Bulk Metallic Glasses after Immersion in Ringer’s Solution. J. Mater. Eng. Perform. 2019, 28, 2365–2377. [Google Scholar] [CrossRef] [Green Version]
- Langelier, B.; Wang, X.; Esmaeili, S. Evolution of precipitation during non-isothermal ageing of an Mg-Ca-Zn alloy with high Ca content. Mater. Sci. Eng. A 2012, 538, 246–251. [Google Scholar] [CrossRef]
- Wang, X.; Du, W.; Wang, Z.; Liu, K.; Li, S. Stable icosahedral phase in Mg44Zn44Gd12 alloy. J. Rare Earths 2012, 30, 503–506. [Google Scholar] [CrossRef]
- Li, D.J.; Zeng, X.Q.; Dong, J.; Zhai, C.Q.; Ding, W.J. Microstructure evolution of Mg-10Gd-3Y-1.2Zn-0.4Zr alloy during heat-treatment at 773 K. J. Alloys Compd. 2009, 468, 164–169. [Google Scholar] [CrossRef]
- Damodaran, V.B.; Murthy, S.N. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Filek, R.; Zhu, X.; Gao, H.; Qiao, L.; Liu, H.; Xie, L.; Wang, Y.; Pan, F.; Hutnik, C.M.L. Bio-modulation of scaring Glaucoma Filtration Surgery using a novel application of coated magnesium. J. Magnes. Alloy. 2020. [Google Scholar] [CrossRef]
- Gungor, A.; Incesu, A. Effects of alloying elements and thermomechanical process on the mechanical and corrosion properties of biodegradable Mg alloys. J. Magnes. Alloy. 2020. [Google Scholar] [CrossRef]
- Chaharmahali, R.; Fattah-alhosseini, A.; Babaei, K. Surface characterization and corrosion behavior of calcium phosphate (Ca-P) base composite layer on Mg and its alloys using plasma electrolytic oxidation (PEO): A review. J. Magnes. Alloy. 2020. [Google Scholar] [CrossRef]
- Yang, H.; Qu, X.; Lin, W.; Chen, D.; Zhu, D.; Dai, K.; Zheng, Y. Enhanced Osseointegration of Zn-Mg Composites by Tuning the Release of Zn Ions with Sacrificial Mg-Rich Anode Design. ACS Biomater. Sci. Eng. 2019, 5, 453–467. [Google Scholar] [CrossRef]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Tie, D.; Guan, R.; Liu, H.; Cipriano, A.; Liu, Y.; Wang, Q.; Huang, Y.; Hort, N. An in vivo study on the metabolism and osteogenic activity of bioabsorbable Mg-1Sr alloy. Acta Biomater. 2016, 29, 455–467. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Wang, H.; Liu, J.; Ouyang, L.; Zhu, M. Destabilizing the dehydriding thermodynamics of MgH2 by reversible intermetallics formation in Mg−Ag−Zn ternary alloys. J. Power Sources 2018, 396, 796–802. [Google Scholar] [CrossRef]
- Wasiur-Rahman, S.; Medraj, M. Critical assessment and thermodynamic modeling of the binary Mg-Zn, Ca-Zn and ternary Mg-Ca-Zn systems. Intermetallics 2009, 17, 847–864. [Google Scholar] [CrossRef]
- Liao, H.; Kim, J.; Lee, T.; Song, J.; Peng, J.; Jiang, B.; Pan, F. Effect of heat treatment on LPSO morphology and mechanical properties of Mg–Zn–Y–Gd alloys. J. Magnes. Alloy. 2020, 4–11. [Google Scholar] [CrossRef]
















| % | Element | Degradation Days | Weight in g. | Complies for Toxicology g/Days | Toxicological Limit in g/Days |
|---|---|---|---|---|---|
| 69.18 | Mg | 150 | 20.75 | 0.140 | 0.700 |
| 4.99 | Zn | 150 | 1.49 | 0.010 | 0.015 |
| 25.83 | Ca | 150 | 7.74 | 0.052 | 0.800 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becerra, L.H.C.; Castro, A.T. Bio-Fabrication and Experimental Validation of an Mg - 25Ca - 5Zn Alloy Proposed for a Porous Metallic Scaffold. Crystals 2021, 11, 1416. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11111416
Becerra LHC, Castro AT. Bio-Fabrication and Experimental Validation of an Mg - 25Ca - 5Zn Alloy Proposed for a Porous Metallic Scaffold. Crystals. 2021; 11(11):1416. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11111416
Chicago/Turabian StyleBecerra, Luis Humberto Campos, and Alejandro Torres Castro. 2021. "Bio-Fabrication and Experimental Validation of an Mg - 25Ca - 5Zn Alloy Proposed for a Porous Metallic Scaffold" Crystals 11, no. 11: 1416. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11111416