Crystal Structures of New Ammonium 5-Aminotetrazolates
Abstract
:1. Introduction
2. Results and Discussion

| Compound | 1 | 2 | 3 |
|---|---|---|---|
| CCDC No. | 1024084 | 1024085 | 1024086 |
| Chemical formula | C4H12N·CH2N5 | C4H14N2S2·2(CH2N5) | C5H14N3·CH2N5 |
| Mr/g·mol−1 | 158.22 | 322.44 | 200.27 |
| Crystal size/mm3 | 0.24 × 0.16 × 0.10 | 0.30 × 0.30 × 0.20 | 0.40 × 0.40 × 0.40 |
| Crystal system | Triclinic | Monoclinic | Monoclinic |
| Space group | P | C2/c | P21/c |
| a/Å | 9.1270(9) | 17.4437(3) | 7.5109(4) |
| b/Å | 10.129(1) | 7.9305(3) | 12.4241(4) |
| c/Å | 10.432(2) | 11.1625(4) | 11.6513(4) |
| α/° | 98.86(1) | – | – |
| β/° | 110.65(2) | 110.13(1) | 106.82(1) |
| γ/° | 106.77(1) | – | – |
| V/Å3 | 828.2(2) | 1449.85(8) | 1040.72(7) |
| Z | 4 | 4 | 4 |
| Dx/g·cm−3 | 1.27 | 1.48 | 1.28 |
| η | 0.09 | 0.38 | 0.09 |
| F(000)/e | 344 | 680 | 432 |
| Temperature/K | 173(2) | 233(2) | 173(2) |
| θmax/° | 25.4 | 26.0 | 28.6 |
| h, k, l range | −10 ≤ h ≤ 9 | −21 ≤ h ≤ 21 | −9 ≤ h ≤ 8 |
| −12 ≤ k ≤ 12 | −9 ≤ k ≤ 9 | −14 ≤ k ≤ 14 | |
| −12 ≤ l ≤ 11 | −13 ≤ l ≤ 13 | −14 ≤ l ≤ 12 | |
| Absorption correction | multi-scan | – | multi-scan |
| Measured reflections | 5042 | 4661 | 6125 |
| Independent reflections (Rint) | 3010 (0.030) | 1418 (0.026) | 1897 (0.021) |
| Observed reflections (I ≥ 2σ(I)) | 2317 | 1285 | 1718 |
| Restraints/parameters | 0/224 | 5/112 | 0/148 |
| R1, wR2 (I ≥ 2σ(I)) | 0.042, 0.091 | 0.031, 0.081 | 0.033, 0.083 |
| R1, wR2 (all data) | 0.062, 0.106 | 0.034, 0.083 | 0.038, 0.087 |
| Goodness of fit | 1.02 | 1.12 | 1.06 |
| Δρmax, Δρmin/e Å−3 | 0.21, −0.19 | 0.21, −0.38 | 0.36, −0.16 |
2.1. Tetramethylammonium 5-Aminotetrazolate (1)

| Compound | Interaction | H···A | D···A | D–H···A | Symmetry Operation A |
|---|---|---|---|---|---|
| 1 | N1–H12···N2 | 2.17(3) | 3.043(3) | 170(2) | 2 − x, 1 − y, 1 − z |
| N6–H61···N3 | 2.25(2) | 3.143(2) | 173(2) | x, y, z | |
| N6–H62···N7 | 2.25(2) | 3.150(2) | 177(2) | 1 − x, 1 − y, −z | |
| N1–H11···N7 | 2.51(2) | 3.385(2) | 159(2) | 1 + x, y, 1 + z | |
| N1–H11···N8 | 2.61(2) | 3.462(3) | 155(2) | 1 + x, y, 1 + z | |
| C9–H···N9 | 2.562 | 3.511(3) | 162.9 | 1 − x, −y, 1 − z | |
| C4–H···N3 | 2.591 | 3.520(2) | 158.3 | x, y, z | |
| C5–H···N4 | 2.592 | 3.474(2) | 149.7 | x, y, z | |
| C3–H···N4 | 2.601 | 3.480(3) | 149.3 | x, y, z | |
| C10–H···N5 | 2.616 | 3.497(3) | 149.6 | x, y, z | |
| C9–H···N10 | 2.644 | 3.542(3) | 152.5 | 1 − x, −y, 1 − z | |
| C7–H···N5 | 2.652 | 3.524(3) | 148.3 | x, y, z | |
| C6–H···N9 | 2.652 | 3.551(2) | 152.7 | −x, −y, −z | |
| 2 | N6–H···N4 | 1.98(2) | 2.870(2) | 175(2) | x, −y, −1/2 + z |
| N6–H···N3 | 2.05(1) | 2.893(2) | 162(2) | x, y, z | |
| N6–H···N5 | 2.07(1) | 2.958(2) | 164(1) | 1/2 − x, −1/2 + y, 5/2 − z | |
| N5–H···N2 | 2.16(2) | 3.037(2) | 174(2) | x, −y, 1/2 + z | |
| N5–H···N1 | 2.20(2) | 3.070(2) | 173(2) | 1 − x, −y, 3 − z | |
| 3 | N8–H···N2 | 2.04(2) | 2.921(2) | 169(1) | 1 − x, −y, 1 − z |
| N8–H···N4 | 2.05(2) | 2.937(1) | 166(1) | 1 − x, −1/2 + y, 3/2 − z | |
| N1–H···N5 | 2.21(2) | 3.080(2) | 169(1) | 1 − x, 1 − y, 1 − z |

| 1 | SG | Dx/g·cm−3 | a/Å | b/Å | c/Å | α/° | β/° | γ/° |
|---|---|---|---|---|---|---|---|---|
| SCXRD | P | 1.27 | 9.1270(9) | 10.129(2) | 10.432(2) | 98.86(1) | 110.65(2) | 106.77(1) |
| PXRD | P | 1.24 | 9.2743(5) | 10.1495(5) | 10.5227(6) | 98.72(1) | 110.81(1) | 106.91(1) |
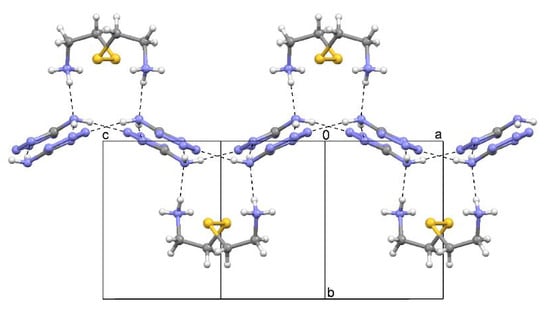
2.2. Cystamine Bis(5-aminotetrazolate) (2)


| 2 | Dx/g·cm−3 | Space Group | a/Å | b/Å | c/Å | β/° |
|---|---|---|---|---|---|---|
| SCXRD | 1.48 | C2/c | 17.4437(3) | 7.9305(3) | 11.1625(4) | 110.131(2) |
| PXRD | 1.47 | C2/c | 17.4964(6) | 7.9400(3) | 11.1614(4) | 110.253(3) |
2.3. 1,1,3,3-Tetramethylguanidinium 5-Aminotetrazolate (3)


| 3 | Dx/g·cm−3 | Space Group | a/Å | b/Å | c/Å | β/° |
|---|---|---|---|---|---|---|
| SCXRD | 1.28 | P21/c | 7.5109(4) | 12.4241(4) | 11.6513(4) | 106.82(1) |
| PXRD | 1.25 | P21/c | 7.6944(2) | 12.5188(5) | 11.5183(6) | 107.03(1) |
2.4. Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)


3. Experimental Section
3.1. Tetramethylammonium 5-Aminotetrazolate (1)
3.2. Cystamine Bis(5-Aminotetrazolate) (2)
3.3. 1,1,3,3-Tetramethylguanidinium 5-Aminotetrazolate (3)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boraei, A.A.A. Acidity constants of some tetrazole compounds in various aqueous-organic solvent media. J. Chem. Eng. Data 2001, 46, 939–943. [Google Scholar] [CrossRef]
- Albert, A.; Goldacre, R.; Phillips, J. The strength of heterocyclic bases. J. Chem. Soc. 1948, 2, 2240–2249. [Google Scholar] [CrossRef]
- Von Denffer, M.; Klapötke, T.M.; Kramer, G.; Spiess, G.; Welch, J.M.; Heeb, G. Improved synthesis and X-ray structure of 5-aminotetrazolium nitrate. Propellants Explos. Pyrotech. 2005, 30, 191–195. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Sabaté, C.M.; Stierstorfer, J. Hydrogen-bonding stabilization in energetic perchlorate salts: 5-Amino-1H-Tetrazolium perchlorate and its adduct with 5-amino-1H-tetrazole. Z. Anorg. Allg. Chem. 2008, 634, 1867–1874. [Google Scholar] [CrossRef]
- Tao, G.-H.; Guo, Y.; Joo, Y.-H.; Twamley, B.; Shreeve, J.M. Energetic nitrogen-rich salts and ionic liquids: 5-Aminotetrazole (AT) as a weak acid. J. Mater. Chem. 2008, 18, 5524–5530. [Google Scholar] [CrossRef]
- Ernst, V.; Klapötke, T.M.; Stierstorfer, J. Alkali salts of 5-aminotetrazole—Structures and properties. Z. Anorg. Allg. Chem. 2007, 633, 879–887. [Google Scholar] [CrossRef]
- Henry, R.A. Salts of 5-aminotetrazole. J. Am. Chem. Soc. 1952, 74, 6303. [Google Scholar] [CrossRef]
- Laus, G.; Kahlenberg, V.; Wurst, K.; Hummel, M.; Schottenberger, H. 1,4-Diazabicyclo[2.2.2]-octane (DABCO) 5-aminotetrazolates. Crystals 2012, 2, 96–104. [Google Scholar] [CrossRef]
- Laus, G.; Kahlenberg, V.; Wurst, K.; Schottenberger, H.; Fischer, N.; Stierstorfer, J.; Klapötke, T.M. Synthesis and crystal structures of new 5,5′-azotetrazolates. Crystals 2012, 2, 127–136. [Google Scholar] [CrossRef]
- Laus, G.; Wurst, K.; Schottenberger, H. Crystal structure of bis(1,3-diaminoimidazolium) 5,5′-azotetrazolate, (C3H7N4)2·(C2N10). Z. Kristallogr. NCS 2012, 227, 293–294. [Google Scholar]
- Fischer, N.; Hüll, K.; Klapötke, T.M.; Stierstorfer, J.; Laus, G.; Hummel, M.; Froschauer, C.; Wurst, K.; Schottenberger, H. 5,5′-Azoxytetrazolates—A new nitrogen-rich dianion and its comparison to 5,5′-azotetrazolate. Dalton Trans. 2012, 41, 11201–11211. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Ogawa, H.; Tanaka, H.; Onishi, A. Blowing Agents of Tetrazoles and Their Derivatives. U.S. Patent US 5,646,292, 8 July 1997. [Google Scholar]
- Zeuner, M.; Pagano, S.; Schnick, W. Nitridosilicates and oxonitridosilicates: From ceramic materials to structural and functional diversity. Angew. Chem. Int. Ed. 2011, 50, 7754–7775. [Google Scholar] [CrossRef]
- Schnick, W.; Huppertz, H. Nitridosilicates—A significant extension of silicate chemistry. Chem. Eur. J. 1997, 3, 679–683. [Google Scholar] [CrossRef]
- Schnick, W.; Schlieper, T.; Huppertz, H.; Köllisch, K.; Orth, M.; Bettenhausen, R.; Schwarze, B.; Lauterbach, R. Nitridosilicates—A significant extension of silicate chemistry. Phosphorus Sulfur Silicon Relat. Elem. 1997, 124–125, 163–172. [Google Scholar] [CrossRef]
- Damavarapu, R.; Klapötke, T.M.; Stierstorfer, J.; Tarantik, K.R. Barium salts of tetrazole derivatives—Synthesis and characterization. Propellants Explos. Pyrotech. 2010, 35, 395–406. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampl, M.; Salchner, R.; Laus, G.; Braun, D.E.; Kahlenberg, V.; Wurst, K.; Fuhrmann, G.; Schottenberger, H.; Huppertz, H. Crystal Structures of New Ammonium 5-Aminotetrazolates. Crystals 2014, 4, 439-449. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst4040439
Lampl M, Salchner R, Laus G, Braun DE, Kahlenberg V, Wurst K, Fuhrmann G, Schottenberger H, Huppertz H. Crystal Structures of New Ammonium 5-Aminotetrazolates. Crystals. 2014; 4(4):439-449. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst4040439
Chicago/Turabian StyleLampl, Martin, Robert Salchner, Gerhard Laus, Doris E. Braun, Volker Kahlenberg, Klaus Wurst, Gerda Fuhrmann, Herwig Schottenberger, and Hubert Huppertz. 2014. "Crystal Structures of New Ammonium 5-Aminotetrazolates" Crystals 4, no. 4: 439-449. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst4040439