Synthesis and Crystal Structures of Azolo[b]1,3,4-Thiadiazinium Bromides
Abstract
:1. Introduction
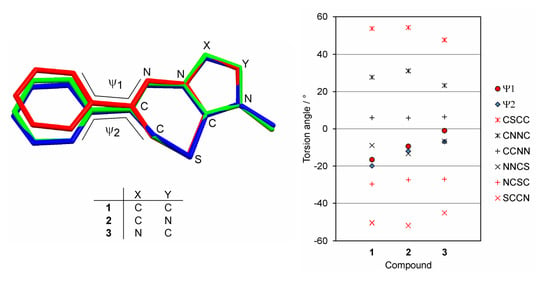
2. Results and Discussion
| Compound | 1 | 2 | 3 |
|---|---|---|---|
| CCDC No. | 1014167 | 1014168 | 1014169 |
| Chemical formula | C12H12N3S·Br·CH4O | C12H14N4S·Br·CH3O0.5 | C11H11N4S·Br |
| Mr | 342.26 | 334.24 | 311.21 |
| Crystal size/mm3 | 0.12 × 0.10 × 0.08 | 0.18 × 0.05 × 0.04 | 0.14 × 0.11 × 0.08 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/n | P21/n | P21/c |
| a/Å | 10.0119(2) | 10.0118(2) | 6.8466(3) |
| b/Å | 7.4539(1) | 7.0742(1) | 6.8350(3) |
| c/Å | 20.1285(4) | 19.6760(4) | 27.0085(9) |
| β/° | 102.829(1) | 99.284(1) | 95.417(2) |
| V/Å3 | 1464.65(5) | 1375.31(4) | 1258.26(9) |
| Z | 4 | 4 | 4 |
| Dx/g cm−3 | 1.55 | 1.61 | 1.64 |
| μ/mm−1 | 2.95 | 3.13 | 3.42 |
| F(000)/e | 696 | 676 | 624 |
| θmax/° | 25.0 | 25.0 | 25.0 |
| h, k, l range | −11 ≤ h ≤ 11 | −11 ≤ h ≤ 11 | −8 ≤ h ≤ 7 |
| −8 ≤ k ≤ 8 | −8 ≤ k ≤ 8 | −7 ≤ k ≤ 8 | |
| −23 ≤ l ≤ 23 | −23 ≤ l ≤ 23 | −30 ≤ l ≤ 32 | |
| Measured reflections | 8091 | 8591 | 6273 |
| Independent reflections (Rint) | 2565 (0.036) | 2422 (0.036) | 2182 (0.039) |
| Observed reflections [I ≥ 2σ(I)] | 2235 | 2030 | 1821 |
| Restraints, parameters | 0, 187 | 6, 180 | 0, 155 |
| R1, wR2 [I ≥ 2σ(I)] | 0.029, 0.079 | 0.030, 0.069 | 0.043, 0.080 |
| R1, wR2 (all data) | 0.034, 0.082 | 0.040, 0.072 | 0.056, 0.083 |
| Goodness of fit | 1.04 | 1.04 | 1.14 |
| Δρmax, Δρmin/e Å−3 | 0.28, −0.25 | 0.42, −0.31 | 0.46, −0.30 |


| Compound | Interaction | H···A | D···A | D–H···A | Symmetry A |
|---|---|---|---|---|---|
| 1 | O1–H···Br | 2.52 | 3.331(4) | 167 | x, y, z |
| C4–HA···Br | 2.86 | 3.590(2) | 132 | 1/2 – x, 1/2 + y, 1/2 – z | |
| C12–HB···Br | 2.87 | 3.796(3) | 161 | 1 – x, –y, 1 – z | |
| C4–HB···Br | 2.90 | 3.749(2) | 146 | x, y, z | |
| C2–H···Br | 2.93 | 3.738(2) | 145 | 1 – x, –y, 1 – z | |
| C12–HC···Br | 2.96 | 3.871(2) | 157 | x, 1 + y, z | |
| 2 | O1 ···Br | – | 2.49(1) | – | x, y, z |
| C2–H···Br | 2.66 | 3.555(3) | 160 | 1 – x, –y, –z | |
| C3–HA···Br | 2.78 | 3.518(3) | 132 | 3/2 – x, –1/2 + y, 1/2 – z | |
| C3–HB···Br | 2.87 | 3.704(3) | 144 | x, y, z | |
| C11–HC···Br | 2.95 | 3.767(4) | 142 | x, –1 + y, z | |
| 3 | C2–H···Br | 2.70 | 3.548(4) | 151 | – x, 1 – y, 1 – z |
| C3–HB···Br | 2.81 | 3.628(4) | 141 | x, –1 + y, z | |
| C11–HC···Br | 2.93 | 3.802(4) | 150 | – x, 1 – y, 1 – z |



3. Experimental Section
3.1. 6-Phenyl-1-Methylimidazo[2,1-b]1,3,4-Thiadiazinium Bromide Methanol Solvate (1)
3.2. 6-Phenyl-1-Methyl-1,2,4-Triazolo[3,4-b]1,3,4-Thiadiazinium Bromide Hemi-Ethanol Solvate (2)
3.3. 6-Phenyl-1-Methyl-1,2,4-Triazolo[3,2-b]1,3,4-Thiadiazinium Bromide (3)
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Becker, H.G.O.; Nagel, D.; Timpe, H.-J. Preparation and reactions of 1-alkyl-4-amino-5-(alkylthio)-s-triazolium salts. J. Prakt. Chem. 1973, 315, 1131–1138. (In German) [Google Scholar] [CrossRef]
- Laus, G.; Klötzer, W. 1-Amino-1H-1,2,4-triazole derivatives. Synthesis 1990, 22, 707–712. [Google Scholar] [CrossRef]
- Kuzmenko, V.V.; Kuzmenko, T.A.; Pozharskii, A.F.; Kryshtalyuk, O.V. 1-Amino-3-alkyl-benzimidazoline-2-thiones(selenones). Khim. Geterotsikl. Soedin. 1990, 1689–1690. (In Russian) [Google Scholar]
- Laus, G.; Kahlenberg, V.; Wurst, K.; Müller, T.; Kopacka, H.; Schottenberger, H. Synthesis and crystal structures of new 1,3-disubstituted imidazoline-2-thiones. Z. Naturforsch. b 2013, 68, 1239–1252. [Google Scholar] [CrossRef]
- Laus, G.; Kahlenberg, V.; Wurst, K.; Schottenberger, H. Synthesis and crystal structures of new 1,4-disubstituted 1,2,4-triazoline-5-thiones. Z. Naturforsch. b 2014, 69, 950–964. [Google Scholar] [CrossRef]
- Alajarin, M.; Molina, P.; de Vega, M.J.P.; Foces-Foces, M.; Cano, F.H.; Claramunt, R.M.; Elguero, J. Aromatic systems with 10π electrons derived from 3a-azapentalene. Part 42. Research in the pyrazolo[5,1-c]-1,2,4-triazole series. Chem. Scr. 1985, 25, 230–238. [Google Scholar]
- Kuzmenko, T.A.; Kuzmenko, V.V.; Kryshtalyuk, O.V.; Pozharsky, A.F. Ring contraction of thia(seleno)diazine to pyrazole ring by reaction of 3-aryl-10-methyl-2H-1,3,4-thia(seleno)diazino-[3,2-a]benzimidazolium salts with bases. Khim. Geterotsikl. Soedin. 1992, 1698–1705. (In Russian) [Google Scholar]
- Kröger, C.-F.; Tenor, E.; Beyer, H. 1,2,4-Triazoles. II Reaction of methyl-substituted thiocarbohydrazides with aliphatic carboxylic acids. Liebigs Ann. Chem. 1961, 643, 121–128. (In German) [Google Scholar] [CrossRef]
- Becker, H.G.O.; Nagel, D.; Timpe, H.-J. C-H-Acid reactions of 1-alkyl-4-amino-1,2,4-triazolium salts. J. Prakt. Chem. 1973, 315, 97–105. (In German) [Google Scholar] [CrossRef]
- Crisostomo-Lucas, C.; Toscano, R.A.; Morales-Morales, D. Synthesis and characterization of new potentially hydrosoluble pincer ligands and their application in Suzuki-Miyaura cross-coupling reactions in water. Tetrahedron Lett. 2013, 54, 3116–3119. [Google Scholar] [CrossRef]
- Wallace, K.J.; Belcher, W.J.; Turner, D.R.; Syed, K.F.; Steed, J.W. Slow anion exchange, conformational equilibria, and fluorescent sensing in Venus Flytrap aminopyridinium-based anion hosts. J. Am. Chem. Soc. 2003, 125, 9699–9715. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Farrugia, L.J. Ortep-3 for Windows—A version of Ortep-III with a Graphical User Interface (GUI). J. Appl. Cryst. 1997, 30. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laus, G.; Wurst, K.; Schottenberger, H. Synthesis and Crystal Structures of Azolo[b]1,3,4-Thiadiazinium Bromides. Crystals 2014, 4, 509-515. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst4040509
Laus G, Wurst K, Schottenberger H. Synthesis and Crystal Structures of Azolo[b]1,3,4-Thiadiazinium Bromides. Crystals. 2014; 4(4):509-515. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst4040509
Chicago/Turabian StyleLaus, Gerhard, Klaus Wurst, and Herwig Schottenberger. 2014. "Synthesis and Crystal Structures of Azolo[b]1,3,4-Thiadiazinium Bromides" Crystals 4, no. 4: 509-515. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst4040509