Preparation, Thermal, and Physical Properties of Perovskite-Type (C3H7NH3)2CdCl4 Crystals
Abstract
:1. Introduction
2. Experimental Section
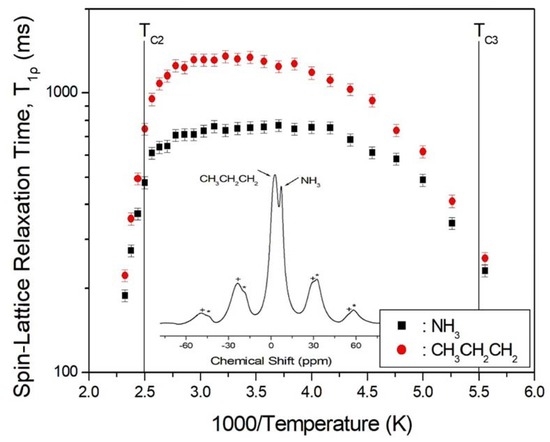
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, C.Q.; Kondo, T.; Sakakura, H.; Kumata, K.; Takahashi, Y.; Ito, R. Optical third-harmonic generation in layered perovskite-type material (C10H21NH3)2PbI4. Solid State Commun. 1991, 79, 245–248. [Google Scholar] [CrossRef]
- Papavassiliou, G.C. Three-and low-dimensional inorganic semiconductors. Prog. Solid State Ch. 1997, 25, 125–270. [Google Scholar] [CrossRef]
- Mitzi, D.B. A layered solution crystal growth technique and the crystal structure of (C6H5C2H4NH3)2PbCl4. J. Solid State Chem. 1999, 145, 694–704. [Google Scholar] [CrossRef]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Strank, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.C.; Mathews, N.; Sun, S.Y.; Lim, S.S.; Lam, Y.M.; Gratzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron- and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Elseman, A.M.; Shalan, A.E.; Sajid, S.; Rashad, M.M.; Hassan, A.M.; Li, M. Copper-substituted lead perovskite materials constructed with different halides for working (CH3NH3)2CuX4-based perovskite solar cells from experimental and theoretical view. ACS Appl. Mater. Inter. 2018, 10, 11699–11707. [Google Scholar] [CrossRef] [PubMed]
- Aramburu, J.A.; Garcia-Fernandez, P.; Mathiesen, N.R.; Garcia-Lastra, J.M.; Moreno, M. Changing the usual interpretation of the structure and ground state of Cu2+ layered perovskite. J. Phys. Chem. C 2018, 122, 5071–5082. [Google Scholar] [CrossRef]
- De Jongh, L.J.; Botterman, A.C.; de Boer, F.R.; Miedema, A.R. Transition temperature of the two-dimensional Heisenberg ferromagnet with s = 1/2. J. Appl. Phys. 1969, 40, 1363–1365. [Google Scholar] [CrossRef]
- Petersson, E.R.; Willett, R.D. Crystal structure of (CH3CH2CH2NH3)2MnCl4. J. Chem. Phys. 1972, 56, 1879–1882. [Google Scholar] [CrossRef]
- Arend, H.; Hofmann, R.; Waldner, F. New phase transition in (CnH2n+1NH3)2MnCl4. Solid State Commun. 1973, 13, 1629–1632. [Google Scholar] [CrossRef]
- Yamazaki, H. Interlayer exchange field in (CnH2n+1NH3)2CuCl4 with n = 1−6 and (C6H5CmH2mNH3)2 CuCl4 with m = 1, 2 determind by parallel pumping experiment. J. Phys. Soc. Jpn. 1976, 41, 1911–1917. [Google Scholar] [CrossRef]
- Jahn, I.R.; Knorr, K.; Ihringer, J. The Jahn-Teller effect and orientational order in (CnH2n+1NH3)2 CuCl4, n = 1,2,3. J. Phys. Condens. Matt. 1989, 1, 6005–6018. [Google Scholar] [CrossRef]
- Lucken, A.; Hagemann, H.; Bill, H. Raman study of the incommensurate layer crystal (CH3CH2CH2NH3)2MnCl4 (=PAMnC) from 10 to 300 K. J. Phys. Condens. Matt. 1991, 3, 5085–5098. [Google Scholar] [CrossRef]
- Narita, N.; Yamada, I. Nonlinear magnetic-susceptibility of two-dimensional magnets (CnH2n+1NH3)2CuCl4 with n = 1, 2 and 3. J. Phys. Soc. Jpn. 1996, 65, 4054–4061. [Google Scholar] [CrossRef]
- Manaka, H.; Yamada, I.; Goto, T. Disappearance of the weak ferromagnetic moment under high pressure observed in the two-dimensional antiferromagnet (C3H7NH3)2CuCl4 through magnetic susceptibility measurements. J. Phys. Soc. Jpn. 2002, 71, 2822–2823. [Google Scholar] [CrossRef]
- Suzuki, K.-I.; Fujimori, H.; Asaji, T.; Ishimaru, S.; Ikeda, R.Z. 1H, 2H and 13C NMR studies of cation dynamics in a layered perovskite-type incommensurate compound (n-C3H7NH3)2CdCl4. Naturforsch 1976, 57, 451. [Google Scholar] [CrossRef]
- Levstik, A.; Filipic, C.; Blinc, R.; Arend, H.; Kind, R. Dielectric properties of (CnH2n+1NH3)2CdCl4 and (NH3(CH2)nNH3)CdCl4 perovskite layer compounds. Solid State Commun. 1976, 20, 127–130. [Google Scholar] [CrossRef]
- Ecolivet, C.; Kusto, W. Brillouin scattering in (C3H7NH3)2CdCl4. Ferroelectrics 1990, 105, 285–290. [Google Scholar] [CrossRef]
- Kusto, W.J.; Rivera, J.-P.; Schmid, H. Birfringence of low-temperature phases of ferroelastic bis(n-propylammonium) tetrachlorocadmate. Ferroelectrics 1992, 15, 191–196. [Google Scholar] [CrossRef]
- Chapuis, G. Study of the first-order phase transition of (C3H7NH3)2CdCl4 at 183K by X-ray diffraction of the two phases. Acta Cryst. B 1978, 34, 1506–1512. [Google Scholar] [CrossRef]
- Doudin, B.; Chapuis, G. Study of the modulated phase of (C3H7NH3)2CdCl4 by single-crystal X-ray diffraction. Acta Cryst. B 1988, 44, 495–502. [Google Scholar] [CrossRef]
- Yoshinari, T.; Matsuyama, T.; Achiwa, N.; Yamaoka, H.; Aoyagi, K. ESR and optical studies on Cl2− in single crystals of (CnH2n+1NH3)2CdCl4 with n = 1,2 and 3. J. Phys. Soc. Jpn. 1987, 56, 3354–3361. [Google Scholar] [CrossRef]
- Blinc, R.; Burgar, M.; Lozar, B.; Seliger, J.; Slak, J.; Rutar, V.; Arend, H.; Kind, R. Proton NMR study of the structural phase transition in perovskite layer compounds: (CnH2n+1NH3)2CdCl4 and (NH3-(CH2)n-NH3)CdCl4. J. Chem. Phys. 1977, 66, 278–287. [Google Scholar] [CrossRef]
- Koenig, J.L. Spectroscopy of Polymers; Elsevier: New York, NY, USA, 1999. [Google Scholar]
- White, M.A.; Granville, N.W.; Davies, N.J.; Staveley, L.A.K. The heat capacity of the layer compounds (CH3CH2CH2NH3)2MnCl4 and (CH3CH2CH2NH3)2CdCl4 from 10 K to 300 K. J. Phys. Chem. Solids 1981, 42, 953–965. [Google Scholar] [CrossRef]
- Lim, A.R. Behavior of H2O surrounding NH4+ and Al3+ in NH4Al(SO4)2·12H2O by 1H MAS NMR, 14N NMR, and 27Al NMR. RSC Adv. 2017, 7, 55276–55281. [Google Scholar] [CrossRef]
- Mcbrierty, V.J.; Packer, K.J. Nuclear Magnetic Resonance in Solid Polymers; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Sakida, S.; Kawamoto, Y. 113Cd MAS and static NMR study of halogenocadmate crystals. J. Phys. Chem. Solids 2002, 63, 151–161. [Google Scholar] [CrossRef]
- Abragam, A. The Principles of Nuclear Magnetism; Oxford University Press: Oxford, UK, 1961. [Google Scholar]
- Abrahams, S.C. Ferroelasticity [ferroelectrics, magnetics and superconductors]. Mater. Res. Bull. 1971, 6, 881–890. [Google Scholar] [CrossRef]
- Salje, E.K.H. Phase Transitions in Ferroelastic and Co-Elastic Crystals; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Aizu, K. Determination of the state parameters and formulation of spontaneous strain for ferroelastics. J. Phys. Soc. Jpn. 1970, 28, 706–716. [Google Scholar] [CrossRef]
- Sapriel, J. Domain-wall orientations in ferroelastics. Phys. Rev. B 1975, 12, 5128–5140. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, A.R.; Kim, S.H. Preparation, Thermal, and Physical Properties of Perovskite-Type (C3H7NH3)2CdCl4 Crystals. Crystals 2019, 9, 108. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9020108
Lim AR, Kim SH. Preparation, Thermal, and Physical Properties of Perovskite-Type (C3H7NH3)2CdCl4 Crystals. Crystals. 2019; 9(2):108. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9020108
Chicago/Turabian StyleLim, Ae Ran, and Sun Ha Kim. 2019. "Preparation, Thermal, and Physical Properties of Perovskite-Type (C3H7NH3)2CdCl4 Crystals" Crystals 9, no. 2: 108. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9020108