Modification of TiO2 Nanowire Arrays with Sn Doping as Photoanode for Highly Efficient Dye-Sensitized Solar Cells
Abstract
:1. Introduction
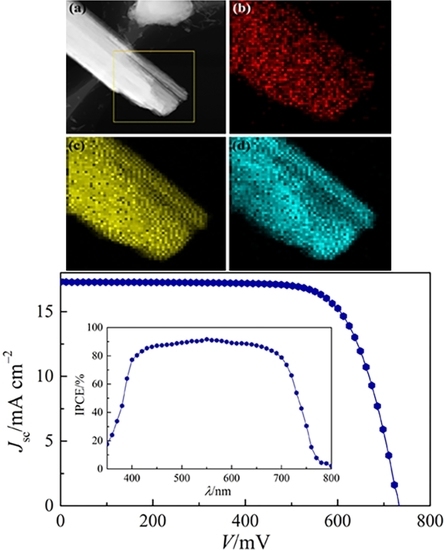
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goetzberger, A.; Hebling, C.; Schock, H.W. Photovoltaic Materials, History, Status and Outlook. Mater. Sci. Eng. R 2003, 40, 1–46. [Google Scholar] [CrossRef]
- Polman, A.; Knight, M.; Garnett, E.C.; Ehrler, B.; Sinke, W.C. Photovoltaic Materials: Present Efficiencies and Future Challenges. Science 2016, 352, aad4424. [Google Scholar] [CrossRef]
- Chapin, D.M.; Fuller, C.S.; Pearson, G.L. A New Silicon p-n Junction Photocell for Converting Solar Radiation into Electrical Power. J. Appl. Phys. 1954, 25, 676–677. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A Low-cost, High-efficiency Solar Cell Based on Dye-sensitized Colloidal TiO2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Fakharuddin, A.; Jose, R.; Brown, T.M.; Fabregat-Santiago, F.; Bisquert, J. A Perspective on the Production of Dye-sensitized Solar Modules. Energy Environ. Sci. 2014, 7, 3952. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Snaith, H.J.; Schmidt-Mende, L. Advances in Liquid-electrolyte and Solid-state Dye-sensitized Solar Cells. Adv. Mater. 2007, 19, 3187–3200. [Google Scholar] [CrossRef]
- Cuce, E.; Young, C.-H.; Riffat, S.B. Thermal performance investigation of heat insulation solar glass: A comparative experimental study. Energy Build. 2015, 86, 595–600. [Google Scholar] [CrossRef]
- Cuce, E.; Riffat, S.B.; Young, C.-H. Thermal insulation, power generation, lighting and energy saving performance of heat insulation solar glass as a curtain wall application in Taiwan: A comparative experimental study. Energ. Convers. Manag. 2015, 96, 31–38. [Google Scholar] [CrossRef]
- Kong, L.; Liu, G.; Gong, J.; Hu, Q.; Dera, P.; Zhang, D.; Liu, Z.; Yang, W.; Zhu, K.; Tang, Y.; et al. Simultaneous band-gap narrowing and carrier-lifetime prolongation of organic-inorganic trihalide perovskites. Proc. Natl. Acad. Sci. USA 2016, 113, 8910–8915. [Google Scholar] [CrossRef]
- Nogueira, A.F.; Longo, C.; De Paoli, M.-A. Polymers in dye sensitized solar cells: Overview and perspectives. Coord. Chem. Rev. 2004, 248, 1455–1468. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, H.; Yu, Q.; Wang, J.; Yu, C.; Wang, J.; Gao, S.; Jiao, S.; Zhang, X.; Wang, P. Single-Layer TiO2 Film Composed of Mesoporous Spheres for High-Efficiency and Stable Dye-Sensitized Solar Cells. ACS Sustain. Chem. Eng. 2018, 6, 3411–3418. [Google Scholar] [CrossRef]
- Yu, Q.; Zhou, D.; Shi, Y.; Si, X.; Wang, Y.; Wang, P. Stable and efficient dye-sensitized solar cells: Photophysical and electrical characterizations. Energy Environ. Sci. 2010, 3, 1722–1725. [Google Scholar] [CrossRef]
- Zhou, D.; Yu, Q.; Cai, N.; Bai, Y.; Wang, Y.; Wang, P. Efficient organic dye-sensitized thin-film solar cells based on the tris(1,10-phenanthroline) cobalt (II/III) redox shuttle. Energy Environ. Sci. 2011, 4, 2030–2034. [Google Scholar] [CrossRef]
- He, B.; Meng, X.; Tang, Q. Low-Cost Counter Electrodes From CoPt Alloys For Efficient DyeSensitized Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 4812–4818. [Google Scholar] [CrossRef]
- Yun, J.H.; Wang, L.; Amal, R.; Ng, Y.H. One-Dimensional TiO2 Nanostructured Photoanodes: From Dye-Sensitised Solar Cells to Perovskite Solar Cells. Energies 2016, 9, 1030. [Google Scholar] [CrossRef]
- Zhang, Q.; Dandeneau, C.S.; Zhou, X.; Cao, G. ZnO Nanostructures for Dye-Sensitized Solar Cells. Adv. Mater. 2009, 21, 4087–4108. [Google Scholar] [CrossRef]
- Fukai, Y.; Kondo, Y.; Mori, S.; Suzuki, E. Highly Efficient Dye-Sensitized SnO2 Solar Cells Having Sufficient Electron Diffusion Length. Electrochem. Commun. 2007, 9, 1439–1443. [Google Scholar] [CrossRef]
- Mahalingam, S.; Abdullah, H. Electron Transport Study of Indium Oxide as Photoanode in DSSCs: A Review. Renew. Sustain. Energy Rev. 2016, 63, 245–255. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Shi, J.; Yu, Y. One-Dimensional Titanium Dioxide Nanomaterials: Nanowires, Nanorods, and Nanobelts. Chem. Rev. 2014, 114, 9346–9384. [Google Scholar] [CrossRef]
- Leung, D.; Fu, X.; Wang, C.; Ni, M.; Leung, M.; Wang, X.; Fu, X. Hydrogen Production over Titania-Based Photocatalysts. ChemSusChem 2010, 3, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Kong, L.; Guo, P.; Stoumpos, C.C.; Hu, Q.; Liu, Z.; Cai, Z.; Gosztola, D.J.; Mao, H.; Kanatzidis, M.G.; et al. Two Regimes of Bandgap Red Shift and Partial Ambient Retention in Pressure-Treated Two-Dimensional Perovskites. ACS Energy Lett. 2017, 2, 2518–2524. [Google Scholar] [CrossRef]
- Liu, G.; Gong, J.; Kong, L.; Schaller, R.D.; Hu, Q.; Liu, Z.; Yan, S.; Yang, W.; Stoumpos, C.C.; Kanatzidis, M.G.; et al. Isothermal pressure-derived metastable states in 2D hybrid perovskites showing enduring bandgap narrowing. Proc. Natl. Acad. Sci. USA 2018, 115, 8076–8081. [Google Scholar] [CrossRef] [PubMed]
- Jose, R.; Thavasi, V.; Ramakrishna, S. Metal oxides for dye-sensitized solar cells. J. Am. Ceram. Soc. 2009, 92, 289–301. [Google Scholar] [CrossRef]
- Wei, H.; Luo, J.; Li, S.; Wang, L. Revealing the Origin of Fast Electron Transfer in TiO2-based Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2016, 138, 8165–8174. [Google Scholar] [CrossRef] [PubMed]
- Roslan, N.; Ya’acob, M.E.; Radzi, M.A.M.; Hashimoto, Y.; Jamaludin, D.; Chen, G. Dye Sensitized Solar Cell (DSSC) greenhouse shading: New insights for solar radiation manipulation. Renew. Sustain. Energy Rev. 2018, 92, 171–186. [Google Scholar] [CrossRef]
- Oekermann, T.; Zhang, D.; Yoshida, T.; Minoura, H. Electron Transport and Back Reaction in Nanocrystalline TiO2 Films Prepared by Hydrothermal Crystallization. J. Phys. Chem. B 2004, 108, 2227–2235. [Google Scholar] [CrossRef]
- Villanueva-Cab, J.; Wang, H.; Oskam, G.; Peter, L.M. Electron Diffusion and Back Reaction in Dye-Sensitized Solar Cells: The Effect of Nonlinear Recombination Kinetics. J. Phys. Chem. Lett. 2010, 1, 748–751. [Google Scholar] [CrossRef] [Green Version]
- Fisher, A.C.; Peter, L.M.; Ponomarev, E.A.; Walker, A.B.; Wijayantha, K.G.U. Intensity Dependence of the Back Reaction and Transport of Electrons in Dye-Sensitized Nanocrystalline TiO2 Solar Cells. J. Phys. Chem. B 2000, 104, 949–958. [Google Scholar] [CrossRef]
- Nelson, J. Continuous-time random-walk model of electron transport in nanocrystalline TiO2 electrodes. Phys. Rev. B 1999, 59, 15374. [Google Scholar] [CrossRef]
- Kang, S.H.; Choi, S.H.; Kang, M.S.; Kim, J.Y.; Kim, H.S.; Hyeon, T.; Sung, Y.E. Nanorod-Based Dye-Sensitized Solar Cells with Improved Charge Collection Efficiency. Adv. Mater. 2008, 20, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.H.; Song, M.Y.; Jang, S.-Y.; Jo, S.M.; Kwak, S.-Y.; Kim, D.Y. Charge Transport Characteristics of High Efficiency Dye-Sensitized Solar Cells Based on Electrospun TiO2 Nanorod Photoelectrodes. J. Phys. Chem. C 2009, 113, 21453–21457. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Fan, J.Q.; Wang, X.; Zhou, W.H.; Du, Z.L.; Wu, S.X. Effect of Highly Ordered Single-Crystalline TiO2 Nanowire Length on the Photovoltaic Performance of Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2011, 3, 4349–4353. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Leung, W.W. Application of a Bilayer TiO2 Nanofiber Photoanode for Optimization of Dye-Sensitized Solar Cells. Adv. Mater. 2011, 23, 4559–4562. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, H.; Liu, B.; Qiu, W.; Zhang, J.; Ran, S.; Huang, H.; Xu, J.; Han, H.; Chen, D.; et al. Transferable and Flexible Nanorod-Assembled TiO2 Cloths for Dye-Sensitized Solar Cells, Photodetectors, and Photocatalysts. ACS Nano 2011, 5, 8412–8419. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhang, X.; Liu, X.; Wang, L.; Wang, C.; Yang, J.; Liu, Y. Growth of Single-Crystalline Rutile TiO2 Nanowire Array on Titanate Nanosheet Film for Dye-Sensitized Solar Cells. J. Mater. Chem. 2012, 22, 6389–6393. [Google Scholar] [CrossRef]
- Xie, J.L.; Guo, C.X.; Li, C.M. Construction of One-Dimensional Nanostructures on Graphene for Efficient Energy Conversion and Storage. Energy Environ. Sci. 2014, 7, 2559–2579. [Google Scholar] [CrossRef]
- Weng, B.; Liu, S.; Tang, Z.-R.; Xu, Y.-J. One-Dimensional Nanostructure Based Materials for Versatile Photocatalytic Applications. RSC Adv. 2014, 4, 12685–12700. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Liu, C.; Ya, J.; Lei, E.; Zhao, W.; Zhao, D.; An, L. TiO2 Photoanode Structure with Gradations in V Concentration for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2011, 3, 1721–1725. [Google Scholar] [CrossRef]
- Liu, J.; Duan, Y.; Zhou, X.; Lin, Y. Influence of VB Group Doped TiO2 on Photovoltaic Performance of Dye-Sensitized Solar Cells. Appl. Surf. Sci. 2013, 277, 231–236. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, N.; You, S.; Liu, Y.; Sebo, B.; Liang, L.; Fang, X.; Liu, W.; Guo, S.; Zhao, X.-Z. Improved Performance of Dye-Sensitized Solar Cells by Trace Amount Cr-doped TiO2 Photoelectrodes. J. Power Sources 2013, 224, 168–173. [Google Scholar] [CrossRef]
- Kim, C.; Kim, K.S.; Kim, H.Y.; Han, Y.S. Modification of a TiO2 Photoanode by Using Cr-doped TiO2 with an Influence on the Photovoltaic Efficiency of a Dye-sensitized Solar Cell. J. Mater. Chem. 2008, 18, 5809–5814. [Google Scholar] [CrossRef]
- Shalan, A.; Rashad, M. Incorporation of Mn2+ and Co2+ to TiO2 nanoparticles and the performance of dye-sensitized solar cells. Appl. Surf. Sci. 2013, 283, 975–981. [Google Scholar] [CrossRef]
- Liau, L.C.-K.; Lin, C.-C. Fabrication and characterization of Fe3+-doped titania semiconductor electrodes with p-n homojunction devices. Appl. Surf. Sci. 2007, 253, 8798–8801. [Google Scholar] [CrossRef]
- Wijayarathna, T.R.C.K.; Aponsu, G.M.L.P.; Ariyasinghe, Y.P.Y.P.; Premalal, E.V.A.; Kumara, G.K.R.; Tennakone, K. A high efficiency indoline-sensitized solar cell based on a nanocrystalline TiO2 surface doped with copper. Nanotechnology 2008, 19, 485703. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-P.; Teng, H. Zinc-doping in TiO2 films to enhance electron transport in dye-sensitized solar cells under low-intensity illumination. Phys. Chem. Chem. Phys. 2009, 11, 9489–9496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Liu, B.; Zhai, J.; Fan, H.; Wang, D.; Lin, Y.; Xie, T. Synthesis of Zn-doped TiO2 microspheres with enhanced photovoltaic performance and application for dye-sensitized solar cells. Electrochim. Acta 2011, 56, 6517–6523. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.-T.; Wang, Z.-S. Effect of metal-doping in TiO2 on fill factor of dye-sensitized solar cells. Appl. Phys. Lett. 2011, 99, 113503. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, P.; Wu, X.; Fu, L.; Zhang, J.; Xu, D. The Origin of Higher Open-Circuit Voltage in Zn-Doped TiO2 Nanoparticle-Based Dye-Sensitized Solar Cells. ChemPhysChem 2012, 13, 3731–3737. [Google Scholar] [CrossRef]
- Zhu, G.; Cheng, Z.; Lv, T.; Pan, L.; Zhao, Q.; Sun, Z. Zn-doped Nanocrystalline TiO2 Films for CdS Quantum Dot Sensitized Solar Cells. Nanoscale 2010, 2, 1229–1232. [Google Scholar] [CrossRef]
- Niaki, A.H.G.; Bakhshayesh, A.M.; Mohammadi, M.R. Double-Layer Dye-Sensitized Solar Cells Based on Zn-doped TiO2 Transparent and Light Scattering Layers: Improving Electron Injection and Light Scattering Effect. Sol. Energy 2014, 103, 210–222. [Google Scholar] [CrossRef]
- Ali, Z.; Park, K.H.; Shakir, I.; Kang, D.J. Ultrathin Conformal Coating and Zn Doping in Nanocrystalline Mesoporous TiO2 Micron-Sized Beads for Highly Efficient Dye Sensitized Solar Cells. Electrochim. Acta 2015, 161, 329–334. [Google Scholar] [CrossRef]
- Qian, J.; Liu, P.; Xiao, Y.; Jiang, Y.; Cao, Y.; Ai, X.; Yang, H. TiO2-Coated Multilayered SnO2 Hollow Microspheres for Dye-sensitized Solar Cells. Adv. Mater. 2009, 21, 3663–3667. [Google Scholar] [CrossRef]
- Breckenridge, R.G.; Hosler, W.R. Electrical Properties of Titanium Dioxide Semiconductors. Phys. Rev. 1953, 91, 793–802. [Google Scholar] [CrossRef]
- Ramasamy, E.; Lee, J. Ordered Mesoporous SnO2-Based Photoanodes for High-Performance Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 22032–22037. [Google Scholar] [CrossRef]
- Parks, G.A. The Isoelectric Points of Solid Oxides, Solid Hydroxides, and Aqueous Hydroxo Complex Systems. Chem. Rev. 1965, 65, 177–198. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, W.; Chen, Z.; Chen, H.; Han, L. Energy Band Tunable TixSn1-xO2 Photoanode for Efficient non-TiO2 Type Dye Sensitized Solar Cells. J. Mater. Chem. A 2013, 1, 8453–8463. [Google Scholar] [CrossRef]
- Gervais, F.; Piriou, B. Temperature Dependence of Transverseand Longitudinal-Optic Modes in TiO2 (Rutile). Phys. Rev. B 1974, 10, 1642–1654. [Google Scholar] [CrossRef]
- Swamy, V.; Muddle, B.C.; Dai, Q. Size-dependent modifications of the Raman spectrum of rutile TiO2. Appl. Phys. Lett. 2006, 89, 163118. [Google Scholar] [CrossRef]
- Lan, T.; Tang, X.; Fultz, B. Phonon Anharmonicity of Rutile TiO2 Studied by Raman Spectrometry and Molecular Dynamics Simulations. Phys. Rev. B 2012, 85, 094305. [Google Scholar] [CrossRef]
- Zhang, Y.; Harris, C.X.; Wallenmeyer, P.; Murowchick, J.; Chen, X. Asymmetric Lattice Vibrational Characteristics of Rutile TiO2 as Revealed by Laser Power Dependent Raman Spectroscopy. J. Phys. Chem. C 2013, 117, 24015–24022. [Google Scholar] [CrossRef]
- Sanjines, R.; Tang, H.; Berger, H.; Gozzo, F.; Margaritondo, G.; Levy, F. Electronic structure of anatase TiO2 oxide. J. Appl. Phys. 1994, 75, 2945–2951. [Google Scholar] [CrossRef]
- Aas, N.; Pringle, T.; Bowker, M. Adsorption and Decomposition of Methanol on TiO2, SrTiO3 and SrO. J. Chem. Soc. Faraday Trans. 1994, 90, 1015–1022. [Google Scholar] [CrossRef]
- Jing, L.; Fu, H.; Wang, B.; Wang, D.; Xin, B.; Li, S.; Sun, J. Effects of Sn Dopant on the Photoinduced Charge Property and Photocatalytic Activity of TiO2 Nanoparticles. Appl. Catal. B 2006, 62, 282–291. [Google Scholar] [CrossRef]
- Li, J.; Zeng, H.C. Hollowing Sn-doped TiO2 Nanospheres via Ostwald Ripening. J. Am. Chem. Soc. 2007, 129, 15839–15847. [Google Scholar] [CrossRef]
- Barr, T.L. Recent Advances in X-ray Photoelectron Spectroscopy Studies of Oxides. J. Vac. Sci. Technol. A 1991, 9, 1793–1805. [Google Scholar] [CrossRef]
- Barr, T.L.; Lishka, M.A.; Chen, L.M.; Mohsenian, M. XPS Valence Band Study of Zeolites and Related Systems. 1. General Chemistry and Structure. J. Am. Chem. Soc. 1988, 110, 7962–7975. [Google Scholar] [CrossRef]
- Teo, S.H.; Zeng, H.C. Surface and Textural Properties of Network-Modified Silica as a Function of Transition Metal Dopant Zirconium. J. Phys. Chem. B 2001, 105, 9093–9100. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Zhang, J.; Chen, H.; Anpo, M. Fe3+-TiO2 photocatalysts prepared by combining sol-gel method with hydrothermal treatment and their characterization. J. Photochem. Photobiol. A Chem. 2006, 180, 196–204. [Google Scholar] [CrossRef]
- Chen, D.; Gao, L. Novel Synthesis of Well-Dispersed Crystalline SnO2 Nanoparticles by Water-in-Oil Microemulsion-Assisted Hydrothermal Process. J. Colloid Interface Sci. 2004, 279, 137–142. [Google Scholar] [CrossRef]
- Xu, R.; Zeng, H.C. Self-Generation of Tiered Surfactant Superstructures for One-Pot Synthesis of Co3O4 Nanocubes and Their Close- and Non-Close-Packed Organizations. Langmuir 2004, 20, 9780–9790. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Lye, M.L.; Zeng, H.C. Large-Scale Synthesis of High-Quality Ultralong Copper Nanowires. Langmuir 2005, 21, 3746–3748. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, H.C. Preparation of Monodisperse Au/TiO2 Nanocatalysts via Self-Assembly. Chem. Mater. 2006, 18, 4270–4277. [Google Scholar] [CrossRef]
- Feng, X.; Shankar, K.; Paulose, M.; Grimes, G.A. Tantalum-Doped Titanium Dioxide Nanowire Arrays for Dye-Sensitized Solar Cells with High Open-Circuit Voltage. Angew. Chem. Int. Ed. 2009, 48, 8095–8098. [Google Scholar] [CrossRef] [PubMed]
- Snaith, H.J.; Ducati, C. SnO2-Based Dye-Sensitized Hybrid Solar Cells Exhibiting Near Unity Absorbed Photon-to-Electron Conversion Efficiency. Nano Lett. 2010, 10, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.H.; Al-Mamun, M.R.; Halder, P.K.; Aziz, M.A. Performance improvement of modified dye-sensitized solar cells. Renew. Sust. Energ. Rev. 2017, 71, 602–617. [Google Scholar] [CrossRef]
- Kay, A.; Grätzel, M. Dye-Sensitized Core-Shell Nanocrystals: Improved Efficiency of Mesoporous Tin Oxide Electrodes Coated with a Thin Layer of an Insulating Oxide. Chem. Mater. 2002, 14, 2930–2935. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, Q.; Wang, J.; Wang, J.; Yu, C.; Abdalla, J.T.; Zeng, Z.; Jiao, S.; Wang, D.; Gao, S. Plasmon-Enhanced Self-Powered UV Photodetectors Assembled by Incorporating Ag@ SiO2 Core-Shell Nanoparticles into TiO2 Nanocube Photoanodes. ACS Sustain. Chem. Eng. 2017, 6, 438–446. [Google Scholar] [CrossRef]
- Ni, S.; Guo, F.; Wang, D.; Liu, G.; Xu, Z.; Kong, L.; Wang, J.; Jiao, S.; Zhang, Y.; Yu, Q.; et al. Effect of MgO Surface Modification on the TiO2 Nanowires Electrode for Self-Powered UV Photodetectors. ACS Sustain. Chem. Eng. 2018, 6, 7265–7272. [Google Scholar] [CrossRef]
- Dou, X.; Sabba, D.; Mathews, N.; Wong, L.H.; Lam, Y.M.; Mhaisalkar, S. Hydrothermal Synthesis of High Electron Mobility Zn-doped SnO2 Nanoflowers as Photoanode Material for Efficient Dye-Sensitized Solar Cells. Chem. Mater. 2011, 23, 3938–3945. [Google Scholar] [CrossRef]
- Kim, C.W.; Suh, S.P.; Choi, M.J.; Kang, Y.S.; Kang, Y.S. Fabrication of SrTiO3-TiO2 heterojunction photoanode with enlarged pore diameter for dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 11820–11827. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Yu, T.; Liu, J.; Zou, Z. Unique Zn-doped SnO2 nano-echinus with excellent electron transport and light harvesting properties as photoanode materials for high performance dye-sensitized solar cell. CrystEngComm 2012, 14, 6462–6468. [Google Scholar] [CrossRef]
- Bisquert, J.; Fabregat-Santiago, F.; Mora-Seró, I.; Garcia-Belmonte, G.; Giménez, S. Electron Lifetime in Dye-Sensitized Solar Cells: Theory and Interpretation of Measurements. J. Phys. Chem. C 2009, 113, 17278–17290. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.S.; Kawauchi, H.; Kashima, T.; Arakawa, H. Significant Influence of TiO2 Photoelectrode Morphology on the Energy Conversion Efficiency of N719 Dye-Sensitized Solar Cell. Coord. Chem. Rev. 2004, 248, 1381–1389. [Google Scholar] [CrossRef]
- Grätzel, M. Recent Advances in Sensitized Mesoscopic Solar Cells. Acc. Chem. Res. 2009, 42, 1788–1798. [Google Scholar] [CrossRef] [PubMed]








| Sample | Jsc (mA cm−2) | Voc (mV) | FF (%) | η (%) | Dye Loading (nmol cm−2) |
|---|---|---|---|---|---|
| TS0 | 13.46 | 748 | 0.741 | 7.46 | 390.4 |
| TS1 | 14.28 | 750 | 0.738 | 7.90 | 382.7 |
| TS2 | 15.84 | 755 | 0.732 | 8.75 | 375.4 |
| TS3 | 11.96 | 760 | 0.776 | 7.05 | 325.2 |
| TS4 | 8.73 | 763 | 0.782 | 5.21 | 288.6 |
| Pin (mW cm−2) | Jsc (mA cm−2) | Voc (mV) | FF | η (%) |
|---|---|---|---|---|
| 100 | 17.28 | 735 | 0.729 | 9.26 |
| 63.63 | 11.39 | 708 | 0.733 | 9.29 |
| 50.33 | 9.28 | 684 | 0.741 | 9.35 |
| 20.29 | 3.85 | 664 | 0.749 | 9.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, S.; Guo, F.; Wang, D.; Jiao, S.; Wang, J.; Zhang, Y.; Wang, B.; Feng, P.; Zhao, L. Modification of TiO2 Nanowire Arrays with Sn Doping as Photoanode for Highly Efficient Dye-Sensitized Solar Cells. Crystals 2019, 9, 113. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9020113
Ni S, Guo F, Wang D, Jiao S, Wang J, Zhang Y, Wang B, Feng P, Zhao L. Modification of TiO2 Nanowire Arrays with Sn Doping as Photoanode for Highly Efficient Dye-Sensitized Solar Cells. Crystals. 2019; 9(2):113. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9020113
Chicago/Turabian StyleNi, Shiming, Fengyun Guo, Dongbo Wang, Shujie Jiao, Jinzhong Wang, Yong Zhang, Bao Wang, Pu Feng, and Liancheng Zhao. 2019. "Modification of TiO2 Nanowire Arrays with Sn Doping as Photoanode for Highly Efficient Dye-Sensitized Solar Cells" Crystals 9, no. 2: 113. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9020113