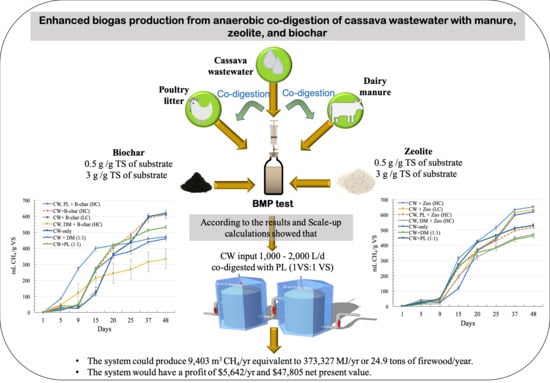
Enhanced Biogas Production of Cassava Wastewater Using Zeolite and Biochar Additives and Manure Co-Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate and Inoculum Collection and Preparation
2.1.1. Cassava Wastewater Substrate
2.1.2. Dairy and Poultry Manure Substrates and Inoculum Source
2.1.3. Biochar and Zeolite Additives
2.2. Experimental Design
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Substrate and Inoculum
3.2. Effect of Livestock Manure Co-Digestion with Cassava Wastewater on Biogas Production
3.2.1. Cumulative CH4 Production Based on VS Addition into the Digestion Reactor
3.2.2. Cumulative CH4 Production Based on the Mass of Substrate Added to the Digestion Reactor
3.2.3. Cumulative CH4 Production Based on Digestion Period
3.3. Impact of Porous Adsorbent on AD of Cassava Wastewater
3.3.1. Zeolite Addition with Cassava Wastewater Digestion
3.3.2. Biochar Addition with Cassava Wastewater Digestion
3.4. Volatile Solids and COD Reduction during Digestion
3.5. Scale-Up Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Glanpracha, N.; Annachhatre, A.P. Anaerobic co-digestion of cyanide containing cassava pulp with pig manure. Bioresour. Technol. 2016, 214, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Panichnumsin, P.; Nopharatana, A.; Ahring, B.; Chaiprasert, P. Production of methane by co-digestion of cassava pulp with various concentrations of pig manure. Biomass Bioenergy 2010, 34, 1117–1124. [Google Scholar] [CrossRef]
- Sun, L.; Wan, S.; Yu, Z.; Wang, Y.; Wang, S. Anaerobic biological treatment of high strength cassava starch wastewater in a new type up-flow multistage anaerobic reactor. Bioresour. Technol. 2012, 104, 280–288. [Google Scholar] [CrossRef] [PubMed]
- FAOStat 2017. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 4 November 2019).
- Achi, C.G.; Coker, A.O.; Sridhar, M.K.C. Utilization and Management of Bioresources. Proceedings of 6th IconSWM; Kumar Ghosh, S., Ed.; Springer: Singapore, 2018; pp. 77–90. [Google Scholar]
- Araujo, I.R.C.; Gomes, S.D.; Tonello, T.U.; Lucas, S.D.; Mari, A.G.; Vargas, R.J. Methane Production from Cassava Starch Wastewater in Packed-Bed Reactor and Continuous Flow. Eng. Agric. 2018, 38, 270–276. [Google Scholar] [CrossRef] [Green Version]
- FAO; IFAD. A review of cassava in Africa with country case studies on Nigeria, Chana, the United Republic of Tanzania, Uganda and Benin. In Processing of the Validation Forum on the Global Cassava Development Strategy; The Food and Agricultural Organization of the United Nations: Rome, Italy; International Fund for Agricultural Development: Rome, Italy, 2005; Volume 2, Available online: http://www.fao.org/3/a-a0154e.pdf (accessed on 4 November 2019).
- Zhang, M.; Xie, L.; Yin, Z.; Khanal, S.K.; Zhou, Q. Biorefinery approach for cassava-based industrial wastes: Current status and opportunities. Bioresour. Technol. 2016, 215, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaewkannetra, P.; Imai, T.; Garcia-Garcia, F.J.; Chiu, T.Y. Cyanide removal from cassava mill wastewater using Azotobactor vinelandii TISTR 1094 with mixed microorganisms in activated sludge treatment system. J. Hazard. Mater. 2009, 172, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, A.; Lansing, S.; Tikekar, R. Impact of metal nanoparticles on biogas production from poultry litter. Bioresour. Technol. 2019, 275, 200–206. [Google Scholar] [CrossRef]
- Paulo, P.L.; Colman-Novaes, T.A.; Obregao, L.D.S.; Boncz, M.A. Anaerobic Digestion of Cassava Wastewater Pre-treated by Fungi. Appl. Biochem. Biotechnol. 2013, 169, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Ubalua, A.O. Cassava wastes: Treatment options and value addition alternatives. Afr. J. Biotechnol. 2007, 6, 2065–2073. [Google Scholar]
- Palma, D.; Fuess, L.T.; de Lima-Model, A.N.; da Conceicao, K.Z.; Cereda, M.P.; Tavares, M.H.F.; Gomes, S.D. Using dolomitic limestone to replace conventional alkalinization in the biodigestion of rapid acidification cassava processing wastewater. J. Clean. Prod. 2018, 172, 2942–2953. [Google Scholar] [CrossRef]
- Amorim, M.C.C.; de S. e Silva, P.T.; Gavazza, S.; Sobrinho, M.A. Viability of rapid startup and operation of UASB reactors for the treatment of cassava wastewater in the semi-arid region of northeastern Brazil. Can. J. Chem. Eng. 2018, 96, 1036–1044. [Google Scholar] [CrossRef]
- Glanpracha, N.; Basnayake, B.M.N.; Rene, E.R.; Lens, P.N.L.; Annachhatre, A.P. Cyanide degradation kinetics during anaerobic co-digestion of cassava pulp with pig manure. Water Sci. Technol. 2018, 77, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.R.; da Silva, F.M.; Tavares, C.R.; Dias Filho, B.P. Bacterial population of a two-phase anaerobic digestion process treating effluent of cassava starch factory. Environ. Technol. 2002, 23, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Qin, Y.; Gadow, S.I.; Ohnishi, A.; Fujimoto, N.; Li, Y.Y. Bio-hythane production from cassava residue by two-stage fermentative process with recirculation. Bioresour. Technol. 2018, 247, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Jiraprasertwong, A.; Maitriwon, K.; Chavadej, S. Production of biogas from cassava wastewater using a three-stage upflow anaerobic sludge blanket (UASB) reactor. Renew. Energy 2019, 130, 191–205. [Google Scholar] [CrossRef]
- Rajagopal, R.; Saady, N.M.C.; Torrijos, M.; Thanikal, J.V.; Hung, Y. Sustainable Agro-Food Industrial Wastewater Treatment Using High Rate Anaerobic Process. Water 2013, 5, 292–311. [Google Scholar] [CrossRef] [Green Version]
- Fagbohungbe, M.O.; Herbert, B.M.J.; Hurst, L.; Ibeto, C.N.; Li, H.; Umani, S.Q.; Semple, K.T. The challenges of anaerobic digestion and the role of biochar in optimizing anaerobic digestion. Waste Manag. 2017, 61, 236–249. [Google Scholar] [CrossRef] [Green Version]
- Mumme, J.; Srocke, F.; Heeg, K.; Werner, M. Use of biochars in anaerobic digestion. Bioresour. Technol. 2014, 164, 189–197. [Google Scholar] [CrossRef]
- Montalvo, S.; Guerrero, L.; Borja, R.; Sanchez, E.; Milan, Z.; Cortes, I.; Rubia, M.A. Application of natural zeolites in anaerobic digestion processes: A review. Appl. Clay Sci. 2012, 58, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Montalvo, S.; Diaz, F.; Guerrero, L.; Sanchez, E.; Borja, R. Effect of particle size and doses of zeolite addition on anaerobic digestion processes of synthetic and piggery wastes. Process Biochem. 2005, 40, 1475–1481. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Yang, Z.; Ding, L.; Zhang, J.; Zhou, J.; Cen, K. Enhanced energy recovery from cassava ethanol wastewater through sequential dark hydrogen, photo hydrogen and methane fermentation combined with ammonium removal. Bioresour. Technol. 2016, 214, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Roopnarain, A.; Adeleke, R. Current status, hurdles and future prospects of biogas digestion technology in Africa. Renew. Sustain. Energy Rev. 2017, 67, 1162–1179. [Google Scholar] [CrossRef]
- Moody, L.B.; Burns, R.T.; Bishop, G.; Sell, S.T.; Spajic, R. Using biochemical methane potential assays to aid in co-substrate selection for co-digestion. Appl. Eng. Agric. 2011, 27, 433–439. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA-AWWA-WEF: Washington, DC, USA, 2005. [Google Scholar]
- Adams, R.C.; MacLean, F.S.; Dixon, J.K.; Bennett, F.M.; Martin, G.I.; Lough, R.C. The Utilization of Organic Wastes in N.Z.: Second Interim Report of the Inter-Departmental Committee. 1951. Available online: http://compost.css.cornell.edu/calc/carbon.html (accessed on 28 November 2019).
- Peres, S.; Monteiro, M.R.; Ferreira, M.L.; do Nascimento, A.F., Jr.; Palha, A.P. Anaerobic Digestion Process for the Production of Biogas from Cassava and Sewage Treatment Plant Sludge in Brazil. BioEnergy Res. 2018, 12, 150–157. [Google Scholar] [CrossRef]
- Witarsa, F.; Lansing, S. Quantifying methane production from psychrophilic anaerobic digestion of separated and unseparated dairy manure. Ecol. Eng. 2015, 78, 95–100. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Murphy, J.D. Unexpectedly low biohydrogen yields in co-fermentation of acid pretreated cassava residue and swine manure. Energy Convers. Manag. 2017, 151, 553–561. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Fazzino, F.; Folino, A.; Paone, E.; Komilis, D. Semi-Continuous Anaerobic Digestion of Orange Peel Waste: Effect of Activated Carbon Addition and Alkaline Pretreatment on the Process. Sustainability 2019, 11, 3386. [Google Scholar] [CrossRef] [Green Version]
- Calabrò, P.S.; Fazzino, F.; Folino, A.; Scibetta, S.; Sidari, R. Improvement of semi-continuous anaerobic digestion of pre-treated orange peel waste by the combined use of zero valent iron and granular activated carbon. Biomass Bioenergy 2019, 129. [Google Scholar] [CrossRef]
- Abouelenien, F.; Namba, Y.; Kosseva, M.R.; Nishio, N.; Nakashimeda, Y. Enhancement of methane production from co-digestion of chicken manure with agricultural wastes. Bioresour. Technol. 2014, 159, 80–87. [Google Scholar] [CrossRef]
- Abouelenien, F.; Namba, Y.; Nishio, N.; Nakashimeda, Y. Dry Co-Digestion of Poultry Manure with Agriculture Wastes. Appl. Biochem. Biotechnol. 2016, 178, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Riaño, B.; Molinuevo, B.; García-González, M.C. Potential for methane production from anaerobic co-digestion of swine manure with winery wastewater. Bioresour. Technol. 2011, 102, 4131–4136. [Google Scholar] [CrossRef] [PubMed]
- Milan, Z.; Sanchez, E.; Weiland, P.; Borja, R.; Martõn, A.; Ilangovan, K. Infuence of different natural zeolite concentrations on the anaerobic digestion of piggery waste. Bioresour. Technol. 2001, 80, 37–43. [Google Scholar] [CrossRef]
- Fagbohungbe, M.O.; Herbert, B.M.; Hurst, L.; Li, H.; Usmani, S.Q.; Semple, K.T. Impact of biochar on the anaerobic digestion of citrus peel waste. Bioresour. Technol. 2016, 216, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.M.; Choi, Y.K.; Kan, E. Effects of dairy manure-derived biochar on psychrophilic, mesophilic and thermophilic anaerobic digestions of dairy manure. Bioresour. Technol. 2018, 250, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, A.; Witarsa, F.; Guo, X.; Yong, L.; Lansing, S. Next generation digestion: Complementing anaerobic digestion (AD) with a novel microbial electrolysis cell (MEC) design. Int. J. Hydrogen Energy 2017, 42, 28681–28689. [Google Scholar] [CrossRef]
- FAO 1998, Woodfuel Flow Study of Phnom Penh, Cambodia. 1998. Available online: http://www.fao.org/3/x5667e/x5667e04.htm (accessed on 28 November 2019).
- World Weather online. Ibadan Monthly Climate Averages. 2019 November 2019. Available online: https://www.worldweatheronline.com/ibadan-weather-averages/oyo/ng.aspx (accessed on 28 November 2019).
- Hassanein, A.; Qiu, L.; Junting, P.; Yihong, G.; Witarsa, F.; Hassanain, A. Simulation and validation of a model for heating underground biogas digesters by solar energy. Ecol. Eng. 2015, 82, 336–344. [Google Scholar] [CrossRef]
- Asikhia, O.; Orugbo, D. Marketing Cost Efficiency of Natural Gas in Nigeria. Pet.-Gas Univ. Ploiesti Bull. 2011, LXIII, 1–13. [Google Scholar]



| Treatment | Cassava Wastewater (CW) (g) | Zeolite (ZEO) (g) | Biochar (B-Char) (g) | Poultry Litter (PL) (g) | Dairy Manure (DM) (g) | Inoculum (g) | Water (g) |
|---|---|---|---|---|---|---|---|
| CW + PL + ZEO (HC) | 28.9 | 3.4 | 0.0 | 0.8 | 0.0 | 92.1 | 28.2 |
| CW + PL + B-Char (HC) | 28.9 | 0.0 | 3.4 | 0.8 | 0.0 | 92.1 | 28.2 |
| CW:PL (1:1) | 28.9 | 0.0 | 0.0 | 0.8 | 0.0 | 92.1 | 28.2 |
| CW:PL (2:1) | 38.6 | 0.0 | 0.0 | 0.5 | 0.0 | 92.1 | 18.9 |
| CW + DM + ZEO (HC) | 28.9 | 3.4 | 0.0 | 0.0 | 4.4 | 92.1 | 24.7 |
| CW:DM (1:1) | 28.9 | 0.0 | 0.0 | 0.0 | 4.4 | 92.1 | 24.7 |
| CW:DM (2:1) | 38.6 | 0.0 | 0.0 | 0.0 | 2.9 | 92.1 | 16.5 |
| CW + ZEO (HC) | 57.8 | 0.3 | 0.0 | 0.0 | 0.0 | 92.1 | 0.1 |
| CW + ZEO (HC) | 57.8 | 1.5 | 0.0 | 0.0 | 0.0 | 92.1 | 0.1 |
| CW + B-Char (LC) | 57.8 | 0.0 | 0.3 | 0.0 | 0.0 | 92.1 | 0.1 |
| CW + B-Char (HC) | 57.8 | 0.0 | 1.5 | 0.0 | 0.0 | 92.1 | 0.1 |
| CW-only | 57.8 | 0.0 | 0.0 | 0.0 | 0.0 | 92.1 | 0.1 |
| Inoculum-only | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 92.1 | 0.0 |
| TS (g/kg) | VS (% TS) | COD (g/L) | pH | TKN (mg N/L) | TP (mg P/L) | C:N Ratio | |
|---|---|---|---|---|---|---|---|
| CW | 17.8 ± 0.7 | 97.2 ± 0.7 | 33.7 ± 0.8 | 5.53 | 375 | 222 | 27.8 |
| PL | 776 ± 1 | 80.0 ± 0.2 | NA | 8.25 | 3675 | 1245 | 13.0 |
| DM | 131 ± 2 | 87.3 ± 0.6 | NA | 7.33 | 3450 | 603 | 15.2 |
| Inoculum | 29.5 ± 0.1 | 73.6 ± 7.0 | 25.1 ± 0.3 | 7.55 | 3050 | 1225 | 3.91 |
| Substrate | VS Reduction (%) | Influent COD (g/L) | COD Reduction (%) | Cumulative CH4 (mL CH4/g VS) | Cumulative CH4 (mL CH4/g Substrate) |
|---|---|---|---|---|---|
| CW-only | 65.5 ± 0.1 a | 29.6 ± 0.4 ab | 40.6 ± 2.9 a | 620 ± 6.0 abc | 10.7 ± 0.1 a |
| CW + B-Char (HC) | 37.5 ± 0.1 b | 41.9 ± 0.7 c | 23.6 ± 7.5 b | 611 ± 27 bc | 10.6 ± 0.5 a |
| CW + B-Char (LC) | 62.7 ± 0.3 a | 34.9 ± 2.4 d | 48.8 ± 5.3 a | 611 ± 16 a | 10.6 ± 0.3 a |
| CW + ZEO (HC) | 66.0 ± 1.9 a | 33.7 ± 0.8 de | 49.2 ± 1.6 a | 653 ± 4 a | 11.3 ± 0.1 ab |
| CW + ZEO (LC) | 66.2 ± 2.6 a | 32.8 ± 1.0 de | 46.2 ± 1.0 a | 634 ± 6 ab | 11.0 ± 0.1 ab |
| CW + PL + B-Char (HC) | 6.88 ± 1.4 c | 41.6 ± 0.6 c | −31.1 ± 1.2 *c | 471 ± 16 d | 15.9 ± 0.5 dce |
| CW + PL + ZEO (HC) | 61.4 ± 0.6 a | 31.6 ± 2.1 ae | 44.0 ± 3.4 a | 518 ± 8 e | 17.4 ± 0.3 dce |
| CW + DM + ZEO (HC) | 57.3 ± 0.2 a | 25.8 ± 0.3 f | 21.9 ± 6.1 b | 473 ± 5 d | 14.2 ± 0.2 dbe |
| CW:PL (1:1) | 63.5 ± 0.6 a | 28.8 ± 2.0 b | 42.7 ± 3.2 a | 531 ± 10 e | 17.9 ± 0.3 c |
| CW:PL (2:1) | 64.8 ± 0.5 a | 28.9 ± 0.3 b | 39.6 ± 4.9 a | 590 ± 6 c | 15.1 ± 0.2 dce |
| CW:DM (1:1) | 59.9 ± 1.0 a | 29.5 ± 0.9 ab | 37.4 ± 0.5 a | 461 ± 17 d | 13.8 ± 0.5 abe |
| CW:DM (2:1) | 63.4 ± 0.3 a | 32.5 ± 1.9 e | 47.8 ± 2.7 a | 522 ± 14 e | 12.6 ± 0.3 abe |
| DM-only | 48.4 ± 5.3 d | 28.0 ± 0.7 bf | 20.9 ± 2.9 b | 100 ± 5 f | 22.9 ± 1.1 f |
| PL-only | 63.0 ± 8.4 a | 28.2 ± 1.3 b | 20.7 ± 0.1 b | 156 ± 3 g | 193 ± 4 g |
| Treatment | Cumulative CH4 in mL CH4/g VS and (% of Total CH4 Production) | ||||
|---|---|---|---|---|---|
| 9 Days | 15 Days | 20 Days | 37 Days | 48 Days | |
| CW-only | 26.8 (4.3%) abc | 118 (19%) a | 364 (58.7%) ab | 598 (96.4%) abc | 620 (100%) abc |
| CW + B-Char (HC) | 39.9 (6.5%) adef | 265 (43.4%) b | 403 (66%) bc | 589 (96.4%) bc | 611 (100%) bc |
| CW + B-Char (LC) | 37.8 (6.2%) acdef | 133 (21.7%) a | 363 (59.4%) a | 591 (96.8%) bc | 611 (100%) bc |
| CW + ZEO (HC) | 39.3 (6%) acdef | 314 (48.1%) c | 425 (65.2%) c | 634 (97.1%) a | 653 (100%) a |
| CW + ZEO (LC) | 31.5 (5%) ace | 189 (29.8%) d | 370 (58.4%) ab | 614 (96.8%) ab | 634 (100%) ab |
| CW + PL + B-Char (HC) | 273 (57.9%) g | 400 (84.8%) e | 419 (88.9%) c | 459 (97.4%) de | 471 (100%) d |
| CW + PL + ZEO (HC) | 43.2 (8.3%) def | 255 (49.2%) b | 370 (71.4%) ab | 497 (95.9%) f | 518 (100%) e |
| CW + DM + ZEO (HC) | 17 (3.6%) b | 197 (41.7%) d | 323 (68.5%) d | 447 (94.7%) de | 473 (100%) d |
| CW:PL (1:1) | 49.8 (9.4%) c | 274 (51.6%) bc | 419 (78.8%) c | 513 (96.5%) f | 531 (100%) e |
| CW:PL (2:1) | 32.6 (5.5%) acde | 263 (44.7%) b | 366 (62%) ba | 571 (96.8%) c | 590 (100%) c |
| CW:DM (1:1) | 46.5 (10.1%) df | 265 (57.6%) b | 353 (76.6%) da | 439 (95.3%) d | 461 (100%) d |
| CW:DM (2:1) | 25.4 (4.9%) bc | 211 (40.5%) d | 336 (64.3%) da | 484 (92.8%) ef | 522 (100%) e |
| DM-only | 34.8 (34.6%) acde | 48.4 (48.2%) f | 62.6 (62.3%) e | 89.1 (88.8%) g | 100 (100%) f |
| PL-only | 92.3 (59%) h | 114 (72.9%) a | 128 (82%) f | 148 (95%) h | 156 (100%) g |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achi, C.G.; Hassanein, A.; Lansing, S. Enhanced Biogas Production of Cassava Wastewater Using Zeolite and Biochar Additives and Manure Co-Digestion. Energies 2020, 13, 491. https://0-doi-org.brum.beds.ac.uk/10.3390/en13020491
Achi CG, Hassanein A, Lansing S. Enhanced Biogas Production of Cassava Wastewater Using Zeolite and Biochar Additives and Manure Co-Digestion. Energies. 2020; 13(2):491. https://0-doi-org.brum.beds.ac.uk/10.3390/en13020491
Chicago/Turabian StyleAchi, Chibueze G., Amro Hassanein, and Stephanie Lansing. 2020. "Enhanced Biogas Production of Cassava Wastewater Using Zeolite and Biochar Additives and Manure Co-Digestion" Energies 13, no. 2: 491. https://0-doi-org.brum.beds.ac.uk/10.3390/en13020491