A Comprehensive Assessment of Bioactive Metabolites, Antioxidant and Antiproliferative Activities of Cyclocarya paliurus (Batal.) Iljinskaja Leaves
Abstract
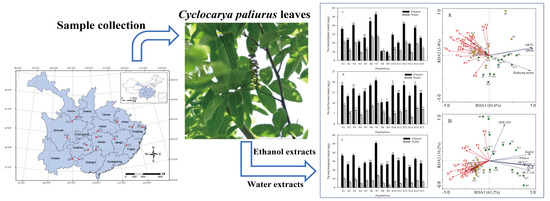
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Extracts
2.3. Content Determination of Bioactive Metabolites
2.4. Antioxidant Assay
2.5. Anticancer Assay
2.5.1. Cell Culture
2.5.2. MTT Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effects of Solvent and Geographical Origin on Bioactive Metabolites
3.2. Effects of Solvent and Geographical Origin on Antioxidant Activity
3.3. Effects of Solvent and Geographical Origin on Anticancer Activity
3.4. Correlation between Phytochemicals and Bioactivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, S.R.; Roh, H.S.; Lee, S.; Park, H.B.; Jang, T.S.; Ko, Y.J.; Baek, K.H.; Kim, K.H. Bioactivity-guided isolation and chemical characterization of antiproliferative constituents from morel mushroom (Morchella esculenta) in human lung adenocarcinoma cells. J. Funct. Foods 2018, 40, 249–260. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; Tavares-da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef]
- Wang, D.D.; Wang, S.; Feng, Y.; Zhang, L.; Li, Z.; Ma, J.; Luo, Y.Q.; Xiao, W. Antitumor effects of Bulbus Fritillariae cirrhosae on Lewis lung carcinoma cells in vitro and in vivo. Ind. Crop. Prod. 2014, 54, 92–101. [Google Scholar] [CrossRef]
- Motta, L.B.; Furlan, C.M.; Santos, D.Y.A.C.; Salatino, M.L.F.; Negri, G.; Carvalho, J.E.D.; Monteiro, P.A.; Ruiz, A.L.T.G.; Caruzo, M.B.; Salatino, A. Antiproliferative activity and constituents of leaf extracts of Croton sphaerogynus Baill. (Euphorbiaceae). Ind. Crop. Prod. 2013, 50, 661–665. [Google Scholar] [CrossRef]
- Elkady, W.M.; Ayoub, I.M. Chemical profiling and antiproliferative effect of essential oils of two Araucaria species cultivated in Egypt. Ind. Crop. Prod. 2018, 118, 188–195. [Google Scholar] [CrossRef]
- Carocho, M.; Calhelha, R.C.; Queiroz, M.R.P.; Bento, A.; Morales, P.; Sokovic, M.; Ferreira, I.C.F.R. Infusions and decoctions of Castanea sativa flowers as effective antitumor and antimicrobial matrices. Ind. Crop. Prod. 2014, 62, 42–46. [Google Scholar] [CrossRef]
- Ashraf, A.; Sarfraz, R.A.; Mahmood, A.; Din, M.U. Chemical composition and in vitro antioxidant and antitumor activities of Eucalyptus camaldulensis Dehn. Leaves. Ind. Crop. Prod. 2015, 74, 241–248. [Google Scholar] [CrossRef]
- Olejnik, A.; Kaczmarek, M.; Olkowicz, M.; Kowalska, K.; Juzwa, W.; Dembczyński, R. ROS-modulating anticancer effects of gastrointestinally digested Ribesnigrum L. fruit extract in human colon cancer cells. J. Funct. Foods 2018, 42, 224–236. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Guan, R.; Chen, J.P.; Yang, D.P.; Zhao, Z.M.; Wang, D.M. Chemical characterization of procyanidins from Spatholobus suberectus and their antioxidative and anticancer activities. J. Funct. Foods 2015, 12, 468–477. [Google Scholar] [CrossRef]
- Dienaitė, L.; Pukalskienė, M.; Matias, A.A.; Pereira, C.V.; Pukalskas, A.; Venskutoni, P.R. Valorization of six Nepeta species by assessing the antioxidant potential, phytochemical composition and bioactivity of their extracts in cell cultures. J. Funct. Foods 2018, 45, 512–522. [Google Scholar] [CrossRef]
- Cao, Y.N.; Fang, S.Z.; Yin, Z.Q.; Fu, X.X.; Shang, X.L.; Yang, W.X.; Yang, H.M. Chemical fingerprint and multicomponent quantitative analysis for the quality evaluation of Cyclocarya paliurus Leaves by HPLC-Q-TOF-MS. Molecules 2017, 22, 1927. [Google Scholar] [CrossRef]
- Xie, J.H.; Wang, Z.J.; Shen, M.Y.; Nie, S.P.; Xie, M.Y. Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocoll. 2016, 53, 7–15. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.N.; Fang, S.Z.; Wang, T.L.; Yin, Z.Q.; Shang, X.L.; Yang, W.X.; Fu, X.X. Antidiabetic effects of Cyclocarya paliurus leaves depends on the contents of antihyperglycemic flavonoids and antihyperlipidemic triterpenoids. Molecules 2018, 23, 1042. [Google Scholar] [CrossRef]
- Yang, Z.W.; Ouyang, K.H.; Zhao, J.; Chen, H.; Xiong, L.; Wang, W.J. Structural characterization and hypolipidemic effect of Cyclocarya paliurus polysaccharide in rat. Int. J. Biol. Macromol. 2016, 91, 1073–1080. [Google Scholar] [CrossRef]
- Xie, J.H.; Liu, X.; Shen, M.Y.; Nie, S.P.; Zhang, H.; Li, C.; Gong, D.M.; Xie, M.Y. Purification, physicochemical characterisation and anticancer activity of a polysaccharide from Cyclocarya paliurus leaves. Food Chem. 2013, 136, 1453–1460. [Google Scholar] [CrossRef]
- Xie, J.H.; Xie, M.Y.; Nie, S.P.; Shen, M.Y.; Wang, Y.X.; Li, C. Isolation, chemical composition and antioxidant activities of a water-soluble polysaccharide from Cyclocarya paliurus (Batal.) Iljinskaja. Food Chem. 2010, 119, 1626–1632. [Google Scholar] [CrossRef]
- Zhou, M.M.; Lin, Y.; Fang, S.Z.; Liu, Y.; Shang, X.L. Phytochemical content and antioxidant activity in aqueous extracts of Cyclocarya paliurus leaves collected from different populations. PeerJ 2019, 7, e6492. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, P.; Zhou, M.M.; Wang, T.L.; Fang, S.Z.; Shang, X.L.; Fu, X.X. Geographic variation in the chemical composition and antioxidant properties of phenolic compounds from Cyclocarya paliurus (Batal.) Iljinskaja Leaves. Molecules 2018, 23, 2440. [Google Scholar] [CrossRef]
- Oueslati, S.; Ksouri, R.; Falleh, H.; Pichette, A.; Abdelly, C.; Legault, J. Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaedafruticosa Forssk. Food Chem. 2012, 132, 943–947. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, S.Z.; Zhou, M.M.; Shang, X.L.; Yang, W.X.; Fu, X.X. Geographic variation in water-soluble polysaccharide content and antioxidant activities of Cyclocarya paliurus leaves. Ind. Crop. Prod. 2018, 121, 180–186. [Google Scholar] [CrossRef]
- Farràs, A.; Cásedas, G.; Les, F.; Terrado, E.M.; Mitjans, M.; López, V. Evaluation of anti-tyrosinase and antioxidant properties of four fern species for potential cosmetic applications. Forests 2019, 10, 179. [Google Scholar] [CrossRef]
- Graça, V.C.; Barros, L.; Calhelha, R.C.; Dias, M.I.; Carvalho, A.M.; Santos-Buelga, C.; Santos, P.F.; Ferreira, I.C.F.R. Chemical characterization and bioactive properties of aqueous and organic extracts of Geranium robertianum L. Food Funct. 2016, 17, 3807–3814. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, C.S.; Chang, W.T.; Wu, M.F.; Cheng, F.T.; Shiau, D.K.; Hsu, C.L. Antioxidant activity and anticancer effect of ethanolic and aqueous extracts of the roots of Ficus beecheyana and their phenolic component. J. Food Drug Anal. 2018, 26, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Alaklabi, A.; Arif, I.A.; Ahamed, A.; Kumar, R.S.; Idhayadhulla, A. Evaluation of antioxidant and anticancer activities of chemical constituents of the Saururus chinensis root extracts. Saudi J. Biol. Sci. 2018, 25, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Heo, B.G.; Park, Y.J.; Park, Y.S.; Bae, J.H.; Cho, J.Y.; Park, K.; Jastrzebski, Z.; Gorinstein, S. Anticancer and antioxidant effects of extracts from different parts of indigo plant. Ind. Crop. Prod. 2014, 56, 9–16. [Google Scholar] [CrossRef]
- Xie, P.J.; Huang, L.X.; Zhang, C.H.; Zhang, Y.L. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure-activity relationships. J. Funct. Foods 2015, 16, 460–471. [Google Scholar] [CrossRef]
- Li, F.M.; Tan, J.; Nie, S.P.; Dong, C.J.; Li, C. The study on determination methods of total flavonoids in Cyclocarya paliurus. Food Sci. Technol. 2006, 4, 34–37. [Google Scholar] [CrossRef]
- Fan, J.P.; He, C.H. Simultaneous quantification of three major bioactive triterpene acids in the leaves of Diospyros kaki by high-performance liquid chromatography method. J. Pharm. Biomed. Anal. 2006, 41, 950–956. [Google Scholar] [CrossRef]
- Lu, Y.; Luthria, D. Influence of postharvest storage, processing, and extraction methods on the analysis of phenolic phytochemicals. Instrum. Method. Anal. Ident. Bioact. Mol. 2014, 1185, 3–31. [Google Scholar] [CrossRef]
- Castro-López, C.; Ventura-Sobrevilla, J.M.; González-Hernández, M.D.; Rojas, R.; Ascacio-Valdés, J.A.; Aguilar, C.N.; Martínez-Ávila, G.C. Impact of extraction techniques on antioxidant capacities and phytochemical composition of polyphenol-rich extracts. Food Chem. 2017, 237, 1139–1148. [Google Scholar] [CrossRef]
- Yakoub, A.R.B.; Abdehedi, O.; Jridi, M.; Elfalleh, W.; Nasri, M.; Ferchichi, A. Flavonoids, phenols, antioxidant, and antimicrobial activities in various extracts from Tossa jute leave (Corchorusolitorus L.). Ind. Crop. Prod. 2018, 118, 206–213. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food Chem. Toxicol. 2013, 55, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Tel-Çayan, G.; Öztürk, M.; Duru, M.E.; Rehman, M.U.; Adhikari, A.; Türkoglu, A.; Choudhary, M.I. Phytochemical investigation, antioxidant and anticholinesterase activities of Ganoderma adspersum. Ind. Crop. Prod. 2015, 76, 749–754. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Luo, M.; Wang, W.; Zhao, C.J.; Gu, C.B.; Zu, Y.G.; Fu, Y.J.; Yao, X.H.; Duan, M.H. Variation of active constituents and antioxidant activity in pyrola (P. incarnata Fisch.) from different sites in Northeast China. Food Chem. 2013, 141, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Gali, L.; Bedjou, F. Antioxidant and anticholinesterase effects of the ethanol extract, ethanol extract fractions and total alkaloids from the cultivated Ruta chalepensis. S. Afr. J. Bot. 2019, 120, 163–169. [Google Scholar] [CrossRef]
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Assessment of the antioxidant and antimutagenic activity of extracts from goji berry of Greek cultivation. Toxicol. Rep. 2018, 5, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Bhebhe, M.; Chipurura, B.; Muchuweti, M. Determination and comparison of phenolic compound content and antioxidant activity of selected local Zimbabwean herbal teas with exotic Aspalathus Linearis. S. Afr. J. Bot. 2015, 100, 213–218. [Google Scholar] [CrossRef]
- Hamid, H.A.; Mutazah, R.; Yusoff, M.M.; Karim, N.A.A.; Razis, A.F.A. Comparative analysis of antioxidant and antiproliferative activities of Rhodomyrtus tomentosa extracts prepared with various solvents. Food Chem. Toxicol. 2017, 108, 451–457. [Google Scholar] [CrossRef]
- Almoulah, N.F.; Voynikov, Y.; Gevrenova, R.; Schohn, H.; Tzanova, T.; Yagi, S.; Thomas, J.; Mignard, B.; Ahmed, A.A.A.; Siddig, M.A.E.; et al. Antibacterial, antiproliferative and antioxidant activity of leaf extracts of selected Solanaceae species. S. Afr. J. Bot. 2017, 112, 368–374. [Google Scholar] [CrossRef]
- Andriani, Y.; Ramli, N.M.; Syamsumir, D.F.; Kassim, M.N.I.; Jaafar, J.; Aziz, N.A.; Marlina, L.; Musa, N.S.; Mohamad, H. Phytochemical analysis, antioxidant, antibacterial and cytotoxicity properties of keys and cores part of Pandanus tectorius fruits. Arab. J. Chem. 2015, 50, 519–521. [Google Scholar] [CrossRef]
- Dahham, S.S.; Al-Rawi, S.S.; Ibrahim, A.H.; Majid, A.S.A.; Majid, A.M.S.A. Antioxidant, anticancer, apoptosis properties and chemical composition of black truffle Terfezia claveryi. Saudi J. Biol. Sci. 2018, 25, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Gouthamchandra, K.; Sudeep, H.V.; Venkatesh, B.J.; Prasad, K.S. Chlorogenic acid complex (CGA7), standardized extract from green coffee beans exerts anticancer effects against cultured human colon cancer HCT-116 cells. Food Sci. Hum. Well. 2017, 6, 147–153. [Google Scholar] [CrossRef]
- Al-Enazi, N.M.; Awaad, A.S.; Zain, M.E.; Alqasoumi, S.I. Antimicrobial, antioxidant and anticancer activities of Laurencia catarinensis, Laurencia majuscule and Padina pavonica extracts. Saudi Pharm. J. 2018, 26, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Siriwatanametanon, N.; Fiebich, B.L.; Efferth, T.; Prieto, J.M.; Heinrich, M. Traditionally used Thai medicinal plants: In vitro anti-inflammatory, anticancer and antioxidant activities. J. Ethnopharmacol. 2010, 130, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lin, Y.L.; Chen, X.R.; Liao, C.C.; Poo, W.K. In vitro assessment of Macleaya cordata crude extract bioactivity and anticancer properties in normal and cancerous human lung cells. Exp. Toxicol. Pathol. 2013, 65, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, R.; Pund, M.; Dawane, A.; Iliyas, S. Evaluation of anticancer, antioxidant, and possible anti-inflammatory properties of selected medicinal plants used in Indian traditional medication. J. Tradit. Complement. Med. 2014, 4, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.J.; Pinho, P.G.D.; Henrique, R.; Pereira, J.A.; Carvalho, M. Further insights into chemical characterization through GC-MS and evaluation for anticancer potential of Dracaena draco leaf and fruit extracts. Food Chem. Toxicol. 2012, 50, 3847–3852. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Guo, X.B.; Liu, Y.; Zhang, M.W.; Zhang, R.F.; Abbasi, A.M.; You, L.J.; Li, T.; Liu, R.H. Comparative assessment of phytochemical profile, antioxidant capacity and anti-proliferative activity in different varieties of brown rice (Oryza sativa L.). LWT-Food Sci. Technol. 2018, 96, 19–25. [Google Scholar] [CrossRef]
- Li, K.; Yang, X.; Hu, X.S.; Han, C.; Lei, Z.F.; Zhang, Z.Y. In vitro antioxidant, immunomodulatory and anticancer activities of two fractions of aqueous extract from Helicteres angustifolia L. root. J. Taiwan Inst. Chem. Eng. 2016, 61, 75–82. [Google Scholar] [CrossRef]
- Yang, H.M.; Yin, Z.Q.; Zhao, M.G.; Jiang, C.H.; Zhang, J.; Pan, K. Pentacyclic triterpenoids from Cyclocarya paliurus and their antioxidant activities in FFA-induced HepG2 steatosis cells. Phytochemistry 2018, 151, 119–127. [Google Scholar] [CrossRef]



| Populations | Ethanol | Water | ||||
|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P1 | P2 | P3 | |
| S1 | 1.23 ± 0.01c | 0.24 ± 0.00b | 0.33 ± 0.02b | 1.43 ± 0.05bc | 0.42 ± 0.03a | 0.03 ± 0.00c |
| S2 | 0.14 ± 0.02i | 0.05 ± 0.01fg | nd | 0.22 ± 0.02hi | 0.09 ± 0.01de | nd |
| S3 | 1.65 ± 0.07a | 0.11 ± 0.00e | 0.19 ± 0.01d | 2.29 ± 0.06a | 0.29 ± 0.01b | nd |
| S4 | 0.15 ± 0.01i | 0.04 ± 0.01g | nd | 0.32 ± 0.04hi | 0.06 ± 0.01de | nd |
| S5 | 0.84 ± 0.03e | 0.16 ± 0.01d | 0.19 ± 0.00d | 0.92 ± 0.01ef | 0.25 ± 0.00b | nd |
| S6 | 0.68 ± 0.01f | 0.15 ± 0.00d | 0.18 ± 0.00d | 1.12 ± 0.01de | 0.27 ± 0.01b | 0.01 ± 0.00d |
| S7 | 1.07 ± 0.02d | 0.31 ± 0.01a | 0.26 ± 0.01c | 1.24 ± 0.18cd | 0.47 ± 0.07a | 0.04 ± 0.00a |
| S8 | 0.49 ± 0.03g | 0.11 ± 0.01e | 0.05 ± 0.01e | 0.66 ± 0.01fg | 0.26 ± 0.01b | nd |
| S9 | 0.26 ± 0.03h | 0.06 ± 0.00f | nd | 0.10 ± 0.01i | 0.03 ± 0.00e | nd |
| S10 | 0.23 ± 0.02hi | 0.05 ± 0.00fg | nd | 0.76 ± 0.01f | 0.20 ± 0.00bc | nd |
| S11 | 0.67 ± 0.06f | 0.19 ± 0.02c | 0.20 ± 0.03d | 0.41 ± 0.01gh | 0.10 ± 0.01de | nd |
| S12 | 1.44 ± 0.01b | 0.21 ± 0.01bc | 0.22 ± 0.01cd | 2.18 ± 0.04a | 0.43 ± 0.02a | nd |
| S13 | 0.59 ± 0.00f | 0.09 ± 0.00e | 0.19 ± 0.01d | 0.43 ± 0.01gh | 0.13 ± 0.00cd | nd |
| S14 | 1.61 ± 0.02a | 0.33 ± 0.01a | 0.47 ± 0.04a | 1.73 ± 0.33b | 0.48 ± 0.08a | 0.04 ± 0.00b |
| S15 | 0.15 ± 0.03i | 0.06 ± 0.01fg | nd | 0.43 ± 0.05gh | 0.20 ± 0.02bc | nd |
| Populations | Ethanol | Water | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F1 | F2 | F3 | F4 | F5 | F6 | F7 | |
| S1 | 3.71 ± 0.02a | 0.76 ± 0.00a | 0.23 ± 0.00fg | 1.55 ± 0.01abc | 0.11 ± 0.00gh | 0.33 ± 0.00a | 2.73 ± 0.02a | 2.30 ± 0.03a | 0.24 ± 0.02b | 0.08 ± 0.00b | 0.95 ± 0.08bc | 0.07 ± 0.01bc | 0.13 ± 0.01a | 0.66 ± 0.27a |
| S2 | 0.20 ± 0.04g | 0.05 ± 0.00fg | 0.02 ± 0.00h | 0.15 ± 0.03h | 0.06 ± 0.00h | 0.02 ± 0.00f | 0.83 ± 0.06f | 0.33 ± 0.05h | 0.05 ± 0.00gh | 0.01 ± 0.00e | 0.25 ± 0.03fg | nd | 0.02 ± 0.00gh | 0.07 ± 0.01hi |
| S3 | 2.26 ± 0.06b | 0.81 ± 0.03a | 0.68 ± 0.02b | 1.06 ± 0.04e | 0.32 ± 0.01d | 0.23 ± 0.01b | 1.69 ± 0.05b | 1.40 ± 0.04c | 0.24 ± 0.01b | 0.06 ± 0.00bc | 0.64 ± 0.02e | 0.06 ± 0.00bc | 0.08 ± 0.00bc | 0.44 ± 0.01c |
| S4 | 0.19 ± 0.01g | 0.04 ± 0.00g | 0.01 ± 0.00h | 0.20 ± 0.08gh | 0.06 ± 0.01h | 0.03 ± 0.00f | 0.16 ± 0.01h | 0.35 ± 0.06h | 0.04 ± 0.00hi | 0.01 ± 0.00e | 0.24 ± 0.03fgh | nd | 0.03 ± 0.01e-h | 0.11 ± 0.02gh |
| S5 | 1.22 ± 0.02e | 0.55 ± 0.01c | 0.28 ± 0.01f | 1.30 ± 0.02cde | 0.20 ± 0.00ef | 0.14 ± 0.00c | 1.07 ± 0.02e | 0.65 ± 0.00ef | 0.12 ± 0.00d | 0.02 ± 0.00e | 0.70 ± 0.00de | nd | 0.04 ± 0.00def | 0.21 ± 0.00e |
| S6 | 2.15 ± 0.04b | 0.63 ± 0.01b | 0.36 ± 0.01e | 1.51 ± 0.03bc | 0.15 ± 0.00fg | 0.07 ± 0.00de | 0.45 ± 0.01g | 1.69 ± 0.02b | 0.28 ± 0.00a | 0.15 ± 0.00a | 1.08 ± 0.01ab | 0.07 ± 0.00bc | 0.04 ± 0.00de | 0.15 ± 0.00fg |
| S7 | 2.01 ± 0.07bc | 0.34 ± 0.01e | 0.16 ± 0.00g | 1.37 ± 0.06cd | 0.14 ± 0.02fgh | 0.17 ± 0.01c | 1.47 ± 0.06c | 1.65 ± 0.04b | 0.18 ± 0.02c | 0.02 ± 0.00de | 1.17 ± 0.02a | 0.06 ± 0.00bc | 0.09 ± 0.01b | 0.56 ± 0.00b |
| S8 | 0.56 ± 0.04f | 0.13 ± 0.01f | 0.05 ± 0.00h | 0.78 ± 0.06f | 0.09 ± 0.00gh | 0.09 ± 0.01d | 0.80 ± 0.06f | 0.42 ± 0.01gh | 0.09 ± 0.00ef | 0.03 ± 0.00de | 0.62 ± 0.01e | 0.06 ± 0.00bc | 0.04 ± 0.00d-g | 0.12 ± 0.00gh |
| S9 | 0.21 ± 0.01g | 0.06 ± 0.00fg | 0.02 ± 0.00h | 0.30 ± 0.01gh | 0.06 ± 0.01h | 0.04 ± 0.00ef | 0.24 ± 0.03h | 0.07 ± 0.00i | 0.02 ± 0.00i | 0.01 ± 0.00e | 0.08 ± 0.01h | nd | 0.02 ± 0.00h | 0.03 ± 0.00i |
| S10 | 0.20 ± 0.03g | 0.05 ± 0.01g | 0.03 ± 0.01h | 0.14 ± 0.02h | 0.07 ± 0.00gh | 0.03 ± 0.00f | 0.16 ± 0.03h | 0.55 ± 0.01fg | 0.05 ± 0.00gh | 0.01 ± 0.00e | 0.32 ± 0.02f | 0.09 ± 0.00b | 0.02 ± 0.00gh | 0.18 ± 0.00ef |
| S11 | 1.44 ± 0.16de | 0.63 ± 0.07bc | 0.40 ± 0.05de | 1.74 ± 0.19ab | 0.25 ± 0.02de | 0.22 ± 0.02b | 1.26 ± 0.14d | 0.46 ± 0.07gh | 0.08 ± 0.01ef | 0.01 ± 0.00e | 0.55 ± 0.09e | 0.05 ± 0.00c | 0.04 ± 0.01de | 0.13 ± 0.02 |
| S12 | 1.84 ± 0.33c | 0.30 ± 0.05e | 0.46 ± 0.03d | 1.19 ± 0.20de | 0.41 ± 0.06c | 0.16 ± 0.03c | 1.82 ± 0.32b | 1.53 ± 0.04bc | 0.11 ± 0.00de | 0.07 ± 0.00b | 0.94 ± 0.02bc | 0.09 ± 0.00b | 0.07 ± 0.00c | 0.55 ± 0.00b |
| S13 | 0.14 ± 0.00g | 0.62 ± 0.02bc | 0.58 ± 0.02c | 0.43 ± 0.01g | 1.43 ± 0.04a | 0.22 ± 0.01b | 1.05 ± 0.03e | 0.07 ± 0.00i | 0.08 ± 0.00ef | 0.05 ± 0.00cd | 0.11 ± 0.00gh | 0.05 ± 0.00c | 0.04 ± 0.00de | 0.14 ± 0.00fg |
| S14 | 1.72 ± 0.07cd | 0.44 ± 0.03d | 1.02 ± 0.07a | 1.77 ± 0.12a | 1.34 ± 0.07b | 0.16 ± 0.02c | 1.72 ± 0.09b | 0.87 ± 0.15d | 0.12 ± 0.02d | 0.15 ± 0.03a | 0.86 ± 0.14cd | 0.19 ± 0.02a | 0.05 ± 0.01d | 0.35 ± 0.06d |
| S15 | 0.20 ± 0.01g | 0.06 ± 0.01fg | 0.02 ± 0.00h | 0.17 ± 0.00gh | 0.07 ± 0.01h | 0.02 ± 0.00f | 1.02 ± 0.06e | 0.73 ± 0.09de | 0.08 ± 0.01fg | 0.02 ± 0.00e | 0.64 ± 0.08e | 0.05 ± 0.00c | 0.03 ± 0.00fgh | 0.17 ± 0.02efg |
| Populations | Ethanol | Water | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T1 | T2 | T5 | T6 | |
| S1 | 3.35 ± 0.03b | 2.00 ± 0.00a | 0.73 ± 0.01ef | 2.38 ± 0.01c | 0.86 ± 0.01b | 0.47 ± 0.00c | 0.16 ± 0.01b | 0.17 ± 0.03cd | nd | 0.07 ± 0.01a |
| S2 | 0.93 ± 0.06ef | 0.21 ± 0.03f | 0.76 ± 0.02ef | 0.32 ± 0.01de | 0.20 ± 0.03ef | 0.14 ± 0.00fg | 0.09 ± 0.01cd | 0.13 ± 0.00e | nd | 0.05 ± 0.00cd |
| S3 | 2.87 ± 0.10b | 1.48 ± 0.04b | 1.91 ± 0.05d | 1.88 ± 0.07c | 0.72 ± 0.02bc | 0.36 ± 0.02d | 0.16 ± 0.01b | 0.16 ± 0.00cde | nd | 0.05 ± 0.00cd |
| S4 | 0.26 ± 0.04g | 0.27 ± 0.01f | Nd | 0.07 ± 0.00e | 0.04 ± 0.01f | 0.05 ± 0.00gh | 0.002 ± 0.0003f | 0.13 ± 0.01e | nd | 0.03 ± 0.00fg |
| S5 | 1.87 ± 0.05c | 1.35 ± 0.04b | 3.11 ± 0.08c | 2.11 ± 0.10c | 0.85 ± 0.07bc | 0.42 ± 0.01cd | 0.24 ± 0.00a | 0.18 ± 0.00abc | 0.04 ± 0.00c | 0.04 ± 0.00efg |
| S6 | 1.51 ± 0.03cd | 0.90 ± 0.02cd | 0.31 ± 0.01fg | 0.89 ± 0.02d | 0.77 ± 0.02bc | 0.71 ± 0.03ab | 0.13 ± 0.00bc | 0.17 ± 0.00e | nd | 0.04 ± 0.00de |
| S7 | 1.50 ± 0.04cd | 0.63 ± 0.02de | 0.77 ± 0.04ef | 0.79 ± 0.02d | 0.31 ± 0.02de | 0.22 ± 0.01ef | 0.17 ± 0.03b | 0.14 ± 0.01de | nd | 0.04 ± 0.00efg |
| S8 | 1.87 ± 0.15c | 0.91 ± 0.06c | 5.33 ± 0.39b | 5.00 ± 0.39b | 0.69 ± 0.04c | 0.37 ± 0.03d | 0.08 ± 0.00d | 0.21 ± 0.00ab | 0.05 ± 0.00b | 0.07 ± 0.00ab |
| S9 | 0.60 ± 0.00fg | 0.14 ± 0.02f | 0.22 ± 0.02fg | 0.23 ± 0.04de | 0.16 ± 0.00ef | 0.02 ± 0.00h | nd | nd | nd | 0.05 ± 0.00cd |
| S10 | 0.23 ± 0.04g | 0.28 ± 0.03f | 0.14 ± 0.02fg | 0.19 ± 0.02de | 0.04 ± 0.01f | 0.05 ± 0.01gh | 0.08 ± 0.01de | 0.18 ± 0.02abc | 0.03 ± 0.00d | 0.04 ± 0.01def |
| S11 | 4.60 ± 0.51a | 2.22 ± 0.23a | 6.09 ± 0.68a | 6.36 ± 0.72a | 1.26 ± 0.14a | 0.80 ± 0.09a | nd | 0.13 ± 0.00e | nd | 0.06 ± 0.00bc |
| S12 | 1.81 ± 0.32c | 1.61 ± 0.25b | Nd | 1.77 ± 0.33c | 0.75 ± 0.13bc | 0.39 ± 0.07cd | 0.16 ± 0.01b | 0.21 ± 0.01a | 0.03 ± 0.00e | 0.03 ± 0.00g |
| S13 | 0.71 ± 0.02efg | 0.34 ± 0.01f | 0.57 ± 0.02fg | 0.46 ± 0.01de | 0.83 ± 0.03bc | 0.66 ± 0.02b | 0.005 ± 0.0008f | nd | nd | 0.03 ± 0.00g |
| S14 | 1.13 ± 0.09de | 0.41 ± 0.02ef | 1.23 ± 0.18e | 0.61 ± 0.05de | 0.24 ± 0.02de | 0.19 ± 0.01ef | 0.04 ± 0.00e | nd | nd | 0.06 ± 0.00bc |
| S15 | 0.79 ± 0.02ef | 0.26 ± 0.05f | 0.46 ± 0.03fg | 0.21 ± 0.00de | 0.40 ± 0.04d | 0.25 ± 0.01e | 0.21 ± 0.04a | 0.17 ± 0.01bc | 0.07 ± 0.00a | 0.04 ± 0.00def |
| Populations | Ethanol | Water | ||||
|---|---|---|---|---|---|---|
| DPPH | ABTS | Reducing Power | DPPH | ABTS | Reducing Power | |
| S1 | 0.52 ± 0.01f | 0.75 ± 0.02cde | 1.10 ± 0.01fg | 1.24 ± 0.01ef | 1.71 ± 0.02ef | 2.48 ± 0.05f |
| S2 | 0.98 ± 0.02c-f | 1.42 ± 0.04b-e | 1.03 ± 0.01g | 2.78 ± 0.05d | 3.68 ± 0.20d | 2.57 ± 0.03f |
| S3 | 0.47 ± 0.00f | 0.64 ± 0.01e | 1.00 ± 0.01g | 1.41 ± 0.01ef | 1.81 ± 0.02ef | 1.95 ± 0.02hi |
| S4 | 1.35 ± 0.01cde | 1.88 ± 0.03bcd | 1.82 ± 0.02bc | 4.44 ± 0.11b | 4.72 ± 0.12c | 3.86 ± 0.00c |
| S5 | 0.81 ± 0.08ef | 1.19 ± 0.01b-e | 1.28 ± 0.02ef | 1.17 ± 0.04ef | 1.55 ± 0.06ef | 1.82 ± 0.06i |
| S6 | 0.40 ± 0.02f | 0.59 ± 0.02e | 0.99 ± 0.01g | 0.88 ± 0.00f | 1.18 ± 0.01f | 1.59 ± 0.01j |
| S7 | 0.92 ± 0.01def | 0.50 ± 0.01e | 1.15 ± 0.01fg | 0.90 ± 0.01f | 1.26 ± 0.01f | 2.09 ± 0.10gh |
| S8 | 5.87 ± 0.64b | 6.68 ± 0.92a | 1.91 ± 0.02bc | 3.65 ± 0.08c | 5.48 ± 0.13b | 4.26 ± 0.04b |
| S9 | 7.23 ± 0.67a | 7.51 ± 1.15a | 3.48 ± 0.16a | 10.65 ± 0.69a | 11.05 ± 0.67a | 5.97 ± 0.11a |
| S10 | 0.67 ± 0.00ef | 0.98 ± 0.02b-e | 1.34 ± 0.01e | 1.39 ± 0.02ef | 2.04 ± 0.15e | 2.18 ± 0.04g |
| S11 | 1.65 ± 0.07c | 2.03 ± 0.09b | 1.77 ± 0.01c | 3.38 ± 0.11c | 5.11 ± 0.45bc | 3.31 ± 0.04d |
| S12 | 0.47 ± 0.01f | 0.74 ± 0.04de | 1.03 ± 0.09g | 1.35 ± 0.02ef | 1.69 ± 0.06ef | 1.45 ± 0.02j |
| S13 | 1.53 ± 0.05cd | 1.90 ± 0.06bc | 1.99 ± 0.15b | 3.93 ± 0.02bc | 3.50 ± 0.09d | 3.27 ± 0.12d |
| S14 | 0.50 ± 0.03f | 0.78 ± 0.02cde | 0.71 ± 0.02h | 1.47 ± 0.02e | 2.05 ± 0.02e | 3.01 ± 0.06e |
| S15 | 0.34 ± 0.00f | 1.44 ± 0.09b-e | 1.54 ± 0.02d | 2.25 ± 0.03d | 1.22 ± 0.01f | 2.91 ± 0.01e |
| Populations | Ethanol | Water | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A549 | HCT-116 | HEK-293 | HeLa | HepG2 | MCF-7 | PANC-1 | A549 | HCT-116 | HEK-293 | HeLa | HepG2 | MCF-7 | PANC-1 | |
| S1 | 0.72 ± 0.03de | 0.66 ± 0.02b | 0.51 ± 0.02b | 0.15 ± 0.01g | 0.41 ± 0.00e | 0.32 ± 0.00def | 0.63 ± 0.00cd | 1.34 ± 0.05cd | 1.04 ± 0.04efg | 8.73 ± 1.17a | 0.68 ± 0.03c | 1.60 ± 0.05ef | 0.72 ± 0.02efg | 1.89 ± 0.09cde |
| S2 | 0.87 ± 0.02b | 0.79 ± 0.00a | 0.40 ± 0.00d | 0.42 ± 0.01a | 0.41 ± 0.00e | 0.33 ± 0.01de | 0.85 ± 0.01a | 3.43 ± 0.52b | 1.71 ± 0.27d | 1.33 ± 0.08bcd | 0.80 ± 0.00bc | 1.52 ± 0.07f | 1.26 ± 0.03bc | 4.82 ± 0.48b |
| S3 | 0.53 ± 0.01j | 0.51 ± 0.00 de | 0.33 ± 0.01fg | 0.28 ± 0.03bcd | 0.34 ± 0.01g | 0.38 ± 0.02bc | 0.54 ± 0.02fg | 2.24 ± 0.46bc | 1.01 ± 0.03efg | 1.05 ± 0.07bcd | 0.22 ± 0.01fg | 2.08 ± 0.03def | 0.75 ± 0.02ef | 2.79 ± 0.29c |
| S4 | 0.96 ± 0.03a | 0.80 ± 0.00a | 0.35 ± 0.02ef | 0.22 ± 0.02def | 0.57 ± 0.01a | 0.41 ± 0.01ab | 0.86 ± 0.03a | - | - | 1.04 ± 0.05bcd | 0.38 ± 0.01ef | 2.72 ± 0.14cd | 1.14 ± 0.08cd | - |
| S5 | 0.60 ± 0.01gh | 0.51 ± 0.01def | 0.68 ± 0.02a | 0.13 ± 0.00g | 0.44 ± 0.00d | 0.35 ± 0.01cd | 0.57 ± 0.01efg | - | 1.85 ± 0.14cd | - | 2.10 ± 0.17a | 3.65 ± 0.57c | 1.45 ± 0.05a | 2.70 ± 0.27cd |
| S6 | 0.35 ± 0.01k | 0.48 ± 0.00efg | 0.11 ± 0.01i | 0.22 ± 0.02ef | 0.38 ± 0.01f | 0.22 ± 0.04g | 0.65 ± 0.01bc | 0.54 ± 0.01d | 0.51 ± 0.02g | - | 0.30 ± 0.002efg | 1.03 ± 0.01f | 0.34 ± 0.01h | 1.08 ± 0.02e |
| S7 | 0.64 ± 0.02fg | 0.47 ± 0.01g | 0.20 ± 0.02h | 0.28 ± 0.00b-e | 0.25 ± 0.00k | 0.38 ± 0.01bc | 0.52 ± 0.02g | 0.87 ± 0.04cd | 0.83 ± 0.03efg | 8.22 ± 0.53a | 0.46 ± 0.02de | 1.24 ± 0.00f | 0.60 ± 0.02g | 1.24 ± 0.03e |
| S8 | 0.77 ± 0.01c | 0.52 ± 0.01d | 0.38 ± 0.01de | 0.34 ± 0.03b | 0.25 ± 0.00k | 0.30 ± 0.01ef | 0.52 ± 0.02g | 1.33 ± 0.07cd | 2.56 ± 0.48b | 2.08 ± 0.24b | 0.39 ± 0.03def | 5.21 ± 0.55b | 1.40 ± 0.11ab | 4.86 ± 0.77b |
| S9 | 0.76 ± 0.00cd | 0.49 ± 0.01efg | 0.43 ± 0.01c | 0.31 ± 0.02bc | 0.25 ± 0.00k | 0.38 ± 0.02bc | 0.54 ± 0.01fg | - | - | 1.16 ± 0.06bcd | 0.96 ± 0.02b | 5.21 ± 0.55b | 1.28 ± 0.07bc | - |
| S10 | 0.62 ± 0.01g | 0.53 ± 0.02d | 0.22 ± 0.00h | 0.29 ± 0.02bc | 0.30 ± 0.00i | 0.30 ± 0.01ef | 0.53 ± 0.01fg | 6.84 ± 1.28a | 1.36 ± 0.17de | 1.25 ± 0.09bcd | 0.20 ± 0.00fg | 5.16 ± 0.25b | 1.22 ± 0.05cd | 2.82 ± 0.19c |
| S11 | 0.71 ± 0.01e | 0.48 ± 0.01fg | 0.49 ± 0.02b | 0.27 ± 0.03cde | 0.32 ± 0.01h | 0.44 ± 0.03a | 0.54 ± 0.00fg | - | 2.34 ± 0.15bc | 1.74 ± 0.15bc | 0.68 ± 0.06c | 9.41 ± 0.37a | 1.16 ± 0.03cd | 9.50 ± 0.46a |
| S12 | 0.56 ± 0.00hij | 0.53 ± 0.01d | 0.21 ± 0.01h | 0.17 ± 0.01fg | 0.47 ± 0.01c | 0.40 ± 0.01b | 0.58 ± 0.01def | 0.90 ± 0.02cd | 0.87 ± 0.01efg | 0.47 ± 0.01d | 0.15 ± 0.01g | 1.20 ± 0.01f | 0.66 ± 0.02fg | 1.44 ± 0.21de |
| S13 | 0.66 ± 0.01f | 0.44 ± 0.00h | 0.36 ± 0.01ef | 0.30 ± 0.05bc | 0.28 ± 0.00j | 0.28 ± 0.01f | 0.55 ± 0.02efg | - | 3.48 ± 0.30a | 0.76 ± 0.01cd | 2.24 ± 0.20a | 5.80 ± 0.95b | - | 9.57 ± 1.04a |
| S14 | 0.55 ± 0.04ij | 0.56 ± 0.01c | 0.31 ± 0.01g | 0.13 ± 0.01g | 0.53 ± 0.01b | 0.29 ± 0.02ef | 0.71 ± 0.03b | 0.97 ± 0.01cd | 0.78 ± 0.01fg | 0.74 ± 0.02cd | 0.60 ± 0.02cd | 1.12 ± 0.02f | 1.08 ± 0.04d | 1.43 ± 0.08e |
| S15 | 0.57 ± 0.01hi | 0.58 ± 0.01c | 0.36 ± 0.01ef | 0.13 ± 0.00g | 0.31 ± 0.00hi | 0.35 ± 0.01cd | 0.60 ± 0.01de | 3.60 ± 0.70b | 1.15 ± 0.04ef | 1.46 ± 0.12bcd | 0.23 ± 0.01fg | 2.68 ± 0.021cde | 0.84 ± 0.03e | 1.10 ± 0.02e |
| Explanatory Variable | Antioxidant | Antiproliferation | ||||
|---|---|---|---|---|---|---|
| Variance Explained% | F Value | p-Value | Variance Explained% | F | p-Value | |
| Tp | 64.1% | 49.974 | 0.002 ** | 29.7% | 11.846 | 0.002 ** |
| Tf | 52.3% | 30.705 | 0.002 ** | 45.2% | 23.132 | 0.002 ** |
| Tt | 36.9% | 16.354 | 0.002 ** | 41.6% | 19.908 | 0.002 ** |
| P1 | 17.9% | 6.087 | 0.014 * | 4.9% | 1.445 | 0.216 |
| P2 | 8.3% | 2.544 | 0.096 | 4.4% | 1.303 | 0.248 |
| P3 | 29.1% | 11.503 | 0.004 ** | 17.4% | 5.879 | 0.008 ** |
| F1 | 33.7% | 14.236 | 0.006 ** | 12.6% | 4.043 | 0.028 * |
| F2 | 28.8% | 11.352 | 0.006 ** | 17.2% | 5.828 | 0.014 * |
| F3 | 24.1% | 8.887 | 0.006 ** | 14.1% | 4.601 | 0.022 * |
| F4 | 29.4% | 11.664 | 0.002 ** | 16.5% | 5.535 | 0.012 * |
| F5 | 8.6% | 2.627 | 0.080 | 8.4% | 2.582 | 0.086 |
| F6 | 20.1% | 7.050 | 0.016 * | 15.1% | 4.972 | 0.018 * |
| F7 | 34.2% | 14.554 | 0.002 ** | 22.9% | 8.313 | 0.004 ** |
| T1 | 16.4% | 5.487 | 0.034 * | 19.9% | 6.976 | 0.010 ** |
| T2 | 18.3% | 6.259 | 0.008 ** | 17.3% | 5.847 | 0.014 * |
| T3 | 2.3% | 0.651 | 0.458 | 9.5% | 2.955 | 0.058 |
| T4 | 3.1% | 0.905 | 0.364 | 11.3% | 3.571 | 0.052 |
| T5 | 14.7% | 4.844 | 0.014 * | 23.0% | 8.360 | 0.004 ** |
| T6 | 14.4% | 4.700 | 0.030 ** | 19.4% | 6.749 | 0.006 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Chen, P.; Lin, Y.; Fang, S.; Shang, X. A Comprehensive Assessment of Bioactive Metabolites, Antioxidant and Antiproliferative Activities of Cyclocarya paliurus (Batal.) Iljinskaja Leaves. Forests 2019, 10, 625. https://0-doi-org.brum.beds.ac.uk/10.3390/f10080625
Zhou M, Chen P, Lin Y, Fang S, Shang X. A Comprehensive Assessment of Bioactive Metabolites, Antioxidant and Antiproliferative Activities of Cyclocarya paliurus (Batal.) Iljinskaja Leaves. Forests. 2019; 10(8):625. https://0-doi-org.brum.beds.ac.uk/10.3390/f10080625
Chicago/Turabian StyleZhou, Mingming, Pei Chen, Yuan Lin, Shengzuo Fang, and Xulan Shang. 2019. "A Comprehensive Assessment of Bioactive Metabolites, Antioxidant and Antiproliferative Activities of Cyclocarya paliurus (Batal.) Iljinskaja Leaves" Forests 10, no. 8: 625. https://0-doi-org.brum.beds.ac.uk/10.3390/f10080625