Age-Dependent Changes in Soil Respiration and Associated Parameters in Siberian Permafrost Larch Stands Affected by Wildfire
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Location
2.2. Field Measurements
2.3. Field Sampling
2.4. Laboratory Analyses and Incubations
Mineral Soil C and N Contents
Microbial Soil BR and SIR
2.5. Data Analyses
3. Results
3.1. Postpyrogenic SR Data Clustering Using PCA
3.2. Temporal and Spatial Variation in SR and Associated Factors, and Their Correlations
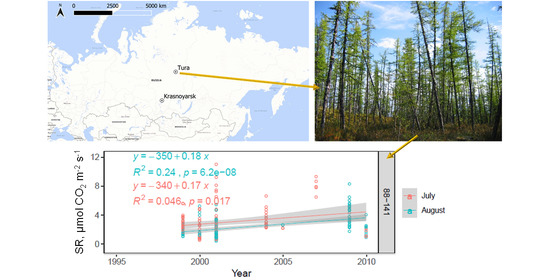
3.3. Long Term (1995–2010) Observation of Postpyrogenic SR and Associated Parameters in Permafrost Larch Forest Successions of Various Ages
4. Discussion
4.1. Spatial and Temporal Variation in SR
4.2. SR Associated Factors, and Their Correlations
4.3. Long-Term (1995–2010) Observation of Postpyrogenic SR Rates and Associated Parameters in Permafrost Larch Forest Successions of Various Ages after Wildfire Disturbance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. A Drop of Humor




References
- Schulze, E.-D.; Beck, E.; Buchmann, N.; Clemens, S.; Müller-Hohenstein, K.; Scherer-Lorenzen, M. Plant Ecology, 2nd ed.; Springer: Heidelberg/Berlin, Germany, 2019. [Google Scholar]
- Masyagina, O.V.; Menyailo, O.V.; Prokushkin, A.S.; Matvienko, A.I.; Makhnykina, A.V.; Evgrafova, S.Y.; Mori, S.; Koike, T.; Prokushkin, S.G. Soil respiration in larch and pine ecosystems of the Krasnoyarsk region (Russian Federation): A latitudinal comparative study. Arab. J. Geosci. 2020, 13, 954. [Google Scholar] [CrossRef]
- Abaimov, A.P.; Prokushkin, S.G.; Sukhovol’skii, V.G.; Ovchinnikova, T.M. Evaluation and prediction of postfire condition of Gmelin larch on permafrost soils in Middle Siberia. Lesovedenie 2004, 2, 3–11. (In Russian) [Google Scholar]
- Abaimov, A.P. Geographical Distribution and Genetics of Siberian Larch Species. In Permafrost Ecosystems. Ecological Studies (Analysis and Synthesis); Osawa, A., Zyryanova, O., Matsuura, Y., Kajimoto, T., Wein, R., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 209. [Google Scholar]
- Stocks, B.J.; Mason, J.A.; Todd, J.B.; Bosch, E.M.; Wotton, B.M.; Amiro, B.D.; Flannigan, M.D.; Hirsch, K.G.; Logan, K.A.; Martell, D.L.; et al. Large forest fires in Canada, 1959–1997. J. Geophys. Res. 2003, 108, 814910. [Google Scholar] [CrossRef]
- Balshi, M.S.; McGuire, A.D.; Zhuang, Q.; Melillo, J.; Kicklighter, D.W.; Kasischke, E.; Wirth, C.; Flannigan, M.; Harden, J.; Clein, J.S.; et al. The role of historical fire disturbance in the carbon dynamics of the pan-boreal region: A process-based analysis. J. Geophys. Res. 2007, 112, G02029. [Google Scholar] [CrossRef] [Green Version]
- Kattenberg, A.; Giorgi, F.; Grassl, H.; Meehl, G.A.; Mitchell, J.F.B.; Stouffer, R.J.; Tokioka, T.; Weaver, A.J.; Wigley, T.M.L. Climate models-projections of future climate. In Climate Change 1995: The Science of Climate Change 2nd Assessment Report of The Intergovernmental Panel on Climate, Change; Houghton, J.T., Meiro Filho, L.G., Callender, B.A., Henris, N., Kattenberg, A., Maskell, K., Eds.; Cambridge University Press: Cambridge, UK, 1996; pp. 285–357. [Google Scholar]
- Permafrost Ecosystems; Osawa, A.; Zyryanova, O.A.; Matsuura, Y.; Kajimoto, T.; Wein, R.W. (Eds.) Ecological Studies; Springer: Dordrecht, The Netherlands, 2010; Volume 209. [Google Scholar]
- Bond-Lamberty, B.; Thompson, A. A global database of soil respiration data. Biogeosciences 2010, 7, 1915–1926. [Google Scholar] [CrossRef] [Green Version]
- Casper, J.K. Greenhouse Gases: Worldwide Impacts; Facts on File; An imprint of InfoBase Publishing, Inc.: New York, NY, USA, 2010; p. 287. [Google Scholar]
- Kharuk, V.I.; Ponomarev, E.I. Spatiotemporal characteristics of wildfire frequency and relative area burned in larch-dominated forests of central Siberia. Russ. J. Ecol. 2017, 48, 507–512. [Google Scholar] [CrossRef]
- NSW Fire and the Environment 2019–20: Summary; Department of Planning, Industry and Environment: Parramatta, Australia, 2020.
- Flannigan, M.D.; Logan, K.A.; Amiro, B.D.; Skinner, W.R.; Stocks, B.J. The future area burned in Canada. Clim. Chang. 2005, 72, 1–16. [Google Scholar] [CrossRef]
- Pechony, O.; Shindell, D.T. Driving forces of global wildfires over the past millennium and the forthcoming century. Proc. Natl. Acad. Sci. USA 2010, 107, 19167–19170. [Google Scholar] [CrossRef] [Green Version]
- Strauss, J.; Schirrmeister, L.; Grosse, G.; Fortier, D.; Hugelius, G.; Knoblauch, C.; Romanovsky, V.; Schädel, C.; Schneider von Deimling, T.; Schuur, E.A.G.; et al. Deep Yedoma permafrost: A synthesis of depositional characteristics and carbon vulnerability. Earth-Sci. Rev. 2017, 172, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Veraverbeke, S.; Rogers, B.M.; Goulden, M.L.; Jandt, R.R.; Miller, C.E.; Wiggins, E.B.; Randerson, J.T. Lightning as a major driver of recent large fire years in North American boreal forests. Nat. Clim. Chang. 2017, 7, 529–534. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. IPCC: Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II, and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Core Writing Team, Eds.; IPCC: Geneva, Switzerland, 2014; 151 p. [Google Scholar]
- Bergner, B.; Johnstone, J.; Treseder, K.K. Experimental warming and burn severity alter soil CO2 flux and soil functional groups in a recently burned boreal forest. Glob. Chang. Biol. 2004, 10, 1996–2004. [Google Scholar] [CrossRef] [Green Version]
- Abbott, B.W.; Jones, J.B. Permafrost collapse alters soil carbon stocks, respiration, CH4, and N2O in upland tundra. Glob. Chang. Biol. 2015, 21, 4570–4587. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Kaduk, J.D.; Balzter, H.; Wooster, M.J.; Mottram, G.N.; Hartley, G.; Lynham, T.J.; Studens, J.; Curry, J.; Stocks, B.J. Soil surface CO2 flux increases with successional time in a fire scar chronosequence of Canadian boreal jack pine forest. Biogeosciences 2010, 7, 1375–1381. [Google Scholar] [CrossRef] [Green Version]
- Köster, E.; Köster, K.; Berninger, F.; Aaltonen, H.; Zhou, X.; Pumpanen, J. Carbon dioxide, methane, and nitrous oxide fluxes from a fire chronosequence in subarctic boreal forests of Canada. Sci. Total Environ. 2017, 601–602, 895–905. [Google Scholar]
- Amiro, B.D.; Barr, A.G.; Barr, J.G.; Black, T.A.; Bracho, R.; Brown, M.; Chen, J.; Clark, K.L.; Davis, K.J.; Desai, A.R.; et al. Ecosystem carbon dioxide fluxes after disturbance in forests of North America. J. Geophys. Res. 2010, 115, G00K02. [Google Scholar] [CrossRef]
- Morishita, T.; Noguchi, K.; Kim, Y.; Matsuura, Y. CO2, CH4, and N2O fluxes of upland black spruce (Picea mariana) forest soils after forest fires of different intensities in interior Alaska. Soil Sci. Plant Nutr. 2015, 61, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Köster, K.; Köster, E.; Orumaa, A.; Parro, K.; Jõgiste, K.; Berninger, F. How time since forest fire affects stand structure, soil physical-chemical properties, and soil CO2 efflux in hemiboreal scots pine forest fire chronosequence? Forests 2016, 7, 201. [Google Scholar]
- Ribeiro-Kumara, C.; Pumpanen, J.; Heinonsalo, J.; Metslaid, M.; Orumaa, A.; Jõgiste, K.; Berninger, F.; Köster, K. Long-term effects of forest fires on soil greenhouse gas emissions and extracellular enzyme activities in a hemiboreal forest. Sci. Total Environ. 2020, 718, 135291. [Google Scholar] [CrossRef]
- Sawamoto, T.; Hatano, R.; Yajima, T.; Takahashi, K.; Isaev, A.P. Soil respiration in Siberian taiga ecosystems with different histories of forest fire. Soil Sci. Plant Nutr. 2000, 46, 31–42. [Google Scholar] [CrossRef]
- Morishita, T.; Masyagina, O.V.; Koike, T.; Matsuura, Y. Soil respiration in arch forests. In Permafrost Ecosystems: Siberian Larch Forests. Ecological Studies; Osawa, A., Zyryanova, O.A., Matsuura, Y., Kajimoto, T., Wein, R.W., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 209, pp. 165–182. [Google Scholar]
- Masyagina, O.V.; Evgrafova, S.Y.; Titov, S.V.; Prokushkin, A.S. Dynamics of soil respiration at different stages of pyrogenic restoration succession with different-aged burns in Evenkia as an example. Rus. J. Ecol. 2015, 46, 27–35. [Google Scholar] [CrossRef]
- Köster, E.; Köster, K.; Berninger, F.; Prokushkin, A.; Aaltonen, H.; Zhou, X.; Pumpanen, J. Changes in fluxes of carbon dioxide and methane caused by fire in Siberian boreal forest with continuous permafrost. J. Environ. Manag. 2018, 228, 405–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meroni, M.; Mollicone, D.; Belelli, L.; Manca, G.; Rosellini, S.; Stivanello, S.; Tirone, G.; Zompanti, R.; Tchebakova, N.; Schulze, E.; et al. Carbon and water exchanges of regenerating forests in central Siberia. For. Ecol. Manag. 2002, 169, 115–122. [Google Scholar] [CrossRef]
- Amiro, B.D.; MacPherson, J.I.; Desjardins, R.L.; Chen, J.M.; Liu, J. Post-fire carbon dioxide fluxes in the western Canadian boreal forest: Evidence from towers, aircraft, and remote sensing. Agric. For. Meteorol. 2003, 115, 91–107. [Google Scholar] [CrossRef]
- Litvak, M.; Miller, S.; Wofsy, S.C.; Goulden, M. Effect of stand age on whole ecosystem CO2 exchange in Canadian boreal forests. J. Geophys. Res. 2003, 108, 822510. [Google Scholar] [CrossRef] [Green Version]
- Schulze, E.D.; Lloyd, J.; Kelliher, F.M.; Wirth, C.; Rebmann, C.; Luhker, B.; Mund, M.; Knohl, A.; Milyukova, I.M.; Schulze, W.; et al. Productivity of forests in the Eurosiberian boreal region and their potential to act as a carbon sink—A synthesis. Glob. Chang. Biol. 1999, 5, 703–722. [Google Scholar] [CrossRef]
- Amiro, B.D. Paired-tower measurements of carbon and energy fluxes following disturbance in the boreal forest. Glob. Chang. Biol. 2001, 7, 253–268. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Wang, C.K.; Gower, S.T. Net primary production and net ecosystem production of a boreal black spruce wildfire chronosequence. Glob. Chang. Biol. 2004, 10, 473–487. [Google Scholar] [CrossRef]
- Amiro, B.D.; Barr, A.G.; Black, T.A.; Iwashita, H.; Kljun, N.; McCaughey, J.H.; Morgenstern, K.; Murayama, S.; Nesic, Z.; Orchansky, A.L.; et al. Carbon, energy and water fluxes at mature and disturbed forest sites, Saskatchewan, Canada. Agric. For. Meteorol. 2006, 136, 237–251. [Google Scholar] [CrossRef]
- Czimczik, C.I.; Trumbore, S.E.; Carbone, M.S.; Winston, G.C. Changing sources of soil respiration with time since fire in a boreal forest. Glob. Chang. Biol. 2006, 12, 957–971. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Zhou, X. Soil Respiration and the Environment.; Academic Press: New York, NY, USA, 2006; p. 333. [Google Scholar]
- Churakova-Sidorova, O.V.; Corona, C.; Fonti, M.V.; Guillet, S.; Saurer, M.; Siegwolf, R.T.W.; Stoffel, M. Recent atmospheric drying in Siberia has not been unprecedented over the last 1,500 years. Sci. Rep. 2020, 10, 15024. [Google Scholar]
- Sofronov, M.A.; Volokitina, A.V.; Shvidenko, A.Z. Wildland fires in the north of Central Siberia. Commonw. For. Rev. 1998, 77, 124–127. [Google Scholar]
- Hiyama, T.; Ueyama, M.; Kotani, A.; Iwata, H.; Nakai, T.; Okamura, M.; Ohta, T.; Harazono, Y.; Petrov, R.E.; Maximov, T.C. Lessons learned from more than a decade of greenhouse gas flux measurements at boreal forests in eastern Siberia and interior Alaska. Polar Sci. 2020, 100607. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, update 2015 International soil classification system for naming soils and creating legends for soil maps. In World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Kajimoto, T.; Matsuura, Y.; Osawa, A.; Prokushkin, A.S.; Sofronov, M.A.; Abaimov, A.P. Root system development of Larix gmelinii trees affected by micro-scale conditions of permafrost soils in central Siberia. Plant Soil 2003, 255, 281–292. [Google Scholar] [CrossRef]
- Anisimov, O.A.; Anokhin, Y.A.; Lavrov, S.A.; Malkova, G.V.; Myach, L.T.; Pavlov, A.V.; Romanovskii, V.A.; Streletskii, D.A.; Kholodov, A.L.; Shiklomanov, N.I. Continental permafrost. In Methods of Assessment of Climate Change Consequences for Physical and Biological Systems; Semenov, S.M., Ed.; Gidrometeoizdat: Moscow, Russia, 2012; pp. 301–359. [Google Scholar]
- Chernoff, H. The use of faces to represent statistical associations. J. Am. Stat. Assoc. 1973, 68, 361–368. [Google Scholar] [CrossRef]
- Warrick, A.W.; Nielsen, D.R. Spatial variability of soil physical properties in the field. In Applications of Soil Physics; Hillel, D., Ed.; Academic Press: New York, NY, USA, 1980; pp. 319–344. [Google Scholar]
- Yanagihara, Y.; Koike, T.; Matsuura, Y.; Mori, S.; Shibata, H.; Satoh, F.; Masyagina, O.V.; Zyryanova, O.A.; Prokushkin, A.S.; Prokushkin, S.G.; et al. Soil respiration rate on the contrasting north- and south-facing slopes of a larch forest in central Siberia. Eurasian J. For. Res. 2000, 1, 19–29. [Google Scholar]
- Knicker, H. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry 2007, 8, 91–118. [Google Scholar] [CrossRef]
- Burke, R.A.; Zepp, R.G.; Tarr, M.A.; Miller, W.L.; Stocks, B.J. Effect of fire on soil-atmosphere exchange of methane and carbon dioxide in Canadian boreal forest sites. J. Geophys. Res. Atmos. 1997, 102, 29289–29300. [Google Scholar] [CrossRef]
- Köster, K.; Berninger, F.; Heinonsalo, J.; Lindén, A.; Köster, E.; Ilvesniemi, H.; Pumpanen, J. The long-term impact of low-intensity surface fires on litter decomposition and enzyme activities in boreal coniferous forests. Int. J. Wildland Fire. 2016, 25, 213–223. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, H.; Sietiö, O.-M.; Pumpanen, J.; Heinonsalo, J.; Köster, K.; Berninger, F. Wildfire effects on soil bacterial community and its potential functions in a permafrost region of Canada. Appl. Soil Ecol. 2020, 156, 103713. [Google Scholar] [CrossRef]
- Wang, C.; Bond-Lamberty, B.; Gower, S.T. Soil surface CO2 flux in a boreal black spruce fire chronosequence. J. Geophys. Res. 2002, 108, 8224. [Google Scholar] [CrossRef]
- Pietikäinen, J.; Fritze, H. Clear-cutting and prescribed burning in coniferous forest: Comparison of effects on soil fungal and total microbial biomass, respiration activity, and nitrification. Soil Biol. Biochem. 1995, 27, 101–109. [Google Scholar] [CrossRef]
- Hu, T.; Sun, L.; Hu, H.; Weise, D.R.; Guo, F. Soil Respiration of the Dahurian Larch (Larix gmelinii) forest and the response to fire disturbance in Da Xing’an Mountains, China. Sci. Rep. 2017, 7, 2967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Phi, P.; Hui, D.; Luo, Y. Global pattern of temperature sensitivity of soil heterotrophic respiration (Q10) and its implications for carbon-climate feedback. J. Geophys. Res. Biogeosci. 2009, 114, G02016. [Google Scholar] [CrossRef] [Green Version]
- Aaltonen, H. Carbon dynamics in forest fire affected permafrost soils. Diss. For. 2020, 288, 54. [Google Scholar] [CrossRef]
- Kajimoto, T. Root system development of larch trees growing on Siberian permafrost. In Permafrost Ecosystems: Siberian Larch Forests. Ecological Studies; Osawa, A., Zyryanova, O.A., Matsuura, Y., Kajimoto, T., Wein, R.W., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 209, pp. 99–122. [Google Scholar]
- Feurdean, A.; Gałka, M.; Florescu, G.; Diaconu, A.-C.; Tant, I.; Kirpotin, S.; Hutchinson, S.M. 2000 years of variability in hydroclimate and carbon accumulation in western Siberia and the relationship with large-scale atmospheric circulation: A multi-proxy peat record. Quat. Sci. Rev. 2019, 226, 105948. [Google Scholar] [CrossRef]
- Masyagina, O.V.; Menyailo, O.V. The impact of permafrost on carbon dioxide and methane fluxes in Siberia: A meta-analysis. Environ. Res. 2020, 182, 109096. [Google Scholar] [CrossRef]






| Site | Vegetation Types (Species, Structure) | Coordinates | Study Period (Number of Measurements) | Vegetation Cover Height (cm) | Stand * | Reference Soil Groups | |||
|---|---|---|---|---|---|---|---|---|---|
| Tree Composition (Age, Years) | Average | Stock (m3 ha−1) | |||||||
| H (m) | DBH (cm) | ||||||||
| T1 | Dwarf-shrub-green moss larch stand with Dushekia understory. Control site of the 2009 burnt area | 64.23N 100.17E, 260 m a.s.l. | 2009 (6), 2010 (6) | 10–25 | 10L (80–100 as of 2010) | 6.9 | 7.2 | 31.0 | CM |
| T2 | Vaccinium-green moss-lichen larch stand. Control site of the 1994 burnt area | 64.32N 100.20E, 230 m a.s.l. | 1995 (40), 1998 (18), 2005 (6), 2007 (10), 2009 (7) | 3–27 | 10L (271 ** as of 2010) | 8.3 | 10.3 | 43.2 | CR |
| T3 | Dwarf-shrub-Sphagnum larch stand | 64.33N 100.27E, 230 m a.s.l. | 1999 (30), 2001 (14) | 10–25 | 10L (104 as of 2006) | 4.1 | 4.1 | 9.6 | HS |
| T4 | Dwarf-shrub-green moss larch stand. Control site of the 1981 burnt area | 64.35N 100.23E, 204 m a.s.l. | 1994 (30); 2005 (4); 2010 (18) | 8–16 | 10L (104 as of 2006) | 7.3 | 6.3 | 26.5 | CM |
| T5 | Vaccinium-Ledum-green moss larch stand. Control site of the 1990 burnt area | 64.32N, 100.27E, 215 m a.s.l. | 1999 (20), 2004 (33), 2005 (6), 2007 (10) | 15–23 | 10L (104 as of 2006) | 7.4 | 5.8 | 32.2 | CM |
| T6 | Dwarf-shrub-lichen-green moss larch stand (near the soil warming experimental plot). Burnt area of 1869 | 64.21N 100.46E, 190 m a.s.l. | 2009 (56), 2010 (14) | 6–25 | 10L (141 as of 2010) | 4.2 | 4.0 | 29.4 | CR |
| T7 | Vaccinium-dry moss-larch stand dominated by Rhytidium rugosum (Hedw.) Kindb. Control site of the landslide of 2009 | 64.33N 100.23E | 2009 (6) | ND | 7L3P (100–200 as of 2010) | 12.0 | 20.0 | 110.0 | CM |
| T8 | Ledum-Vaccinium-green moss larch stand dominated by Pleurozium schreberi (Brid.) Mitt. Control site of the landslide of 2001 | 64.18N 100.53E | 2007 (6), 2008 (6) | 2–4 | 10L (150 as of 2007) | 13.0 | 14.0 | 50.0 | CM |
| T9 | Ledum-Vaccinium-green moss larch stand dominated by Pleurozium schreberi (Brid.) Mitt. Control site of the landslide of 1972 | 64.18N 100.68E | 2007 (6), 2008 (6) | 3.5–5 | 10L (90–150 as of 2007) | 9.0 | 10.0 | 90.0 | CM |
| T10 | Dwarf shrub-Carex-green moss larch stand with Salix spp. understory (Tura Station) | 64.29N, 100.20E | 1999 (29), 2000(71), 2001 (160), 2010 (6) | 5–22.5 | 9L1B (105 as of 2013) | 4.9 | 4.4 | 42.6 | CM |
| T11 | Dwarf-shrub-green moss larch stand. South-exposed control plot of the 1993 burnt area (S93) | 64.33N, 100.22E, 160 m a.s.l. | 2008 (7), 2009 (6) | 2–8 | 10L (188 as of 2008) | 10.5 | 11.0 | 39.0 | CM |
| T12 | Vaccinium-green moss-larch stand. Northern exposition of the control plot of the burnt area of 1993 (N93) | 64.33N, 100.22E, 160 m a.s.l. | 2008 (9), 2009 (6) | 5–10 | 10L (228–275 as of 2008) | 6.9 | 7.8 | 32.0 | CR |
| T13 | Vaccinium-Ledum-green moss larch stand. North-facing slope of the control plot of the 1990 burnt area (N90) | 64.33N, 100.26E, 176 m a.s.l. | 1999 (21), 2004 (34), 2007 (11) | 9–11 | 10L (104 as of 2006) | 7.7 | 5.8 | 41.7 | CR |
| T14 | Dwarf-shrubs-larch stand. PP1-2 | 64.33N, 100.25E | 1999 (20) | 2.5–6 | 9L1B (83 as of 2001) | 6.1 | 6.2 | 10.7 | CM |
| T15 | Burnt area of 2005 | 64.53N, 100.24E | 2005 (6) | - | 10L (1 as of 2006) | 0 | 0 | - | CR |
| T16 | Dwarf shrub-lichen-green moss larch stand, control plot of the burnt area of 2005 | 64.53N, 100.25E | 2005 (6) | ND | 10L (154 as of 2006) | 9.6 | 10.6 | 53.1 | CR |
| T17 | Dwarf shrub-green moss-larch stand with Dushekia understory, burnt area of 2009, ground wildfire | 64.23N 100.17E, 260 m a.s.l. | 2009 (6), 2010 (21) | 3–10 | 10L (1 as of 2010) | 0.2 | 0.0 | - | CM |
| T18 | Vaccinium-green moss-lichen-larch stand, burnt area of 1994, a steady ground fire of moderate intensity | 64.32N 100.20E, 230 m a.s.l. | 1995 (40), 1996(7), 1997 (21), 1998 (21), 1999 (10), 2000 (12), 2005 (6), 2007 (10), 2009 (7), 2010 (24) | 2–10 | 10L (16 as of 2010) | 1.4 | 0.46 | 6.0 | CR |
| T19 | Dwarf-shrub-green moss larch stand, burnt area of 1981, strong rapid ground fire | 64.35N 100.23E, 203 m a.s.l. | 2005 (6), 2010 (24) | 4–7 | 10L (29 as of 2010) | 4.1 | 2.4 | 22.2 | CR |
| T20 | Vaccinium-Ledum-green moss-larch stand, burnt area of 1990, strong running ground fire | 64.33N 100.35E | 2000 (6), 2004 (8), 2005 (6), 2007 (8) | 8–10 | 10L (26 as of 2010) | 2.9 | 1.5 | 14.4 | CM |
| T21 | Ledum-Vaccinium-green moss-larch stand, burnt area of 1978, strong running ground fire | 64.34N, 100.27E | 2000 (6) | 13–20 | 10L (32 as of 2010) | 4.8 | 2.7 | 32.0 | CR |
| T22 | Dwarf-shrub-green moss shrubland with Salix spp. and D. fruticosa, sparse Larix, burnt area of 1993, a ground fire of high intensity | 64.33 N, 100.22E | 1995 (30) | ND | 0L (17 as of 2010) | 0 | 0 | - | CR |
| T23 | Ledum-Vaccinium-green moss-larch stand with Dushekia understory, burnt area of 1951, strong running ground fire after winter selective cutting | 64.30N, 100.22E | 2005 (6) | ND | 10L (55 as of 2006) | 9.5 | 6.8 | 73.3 | CM |
| Variable | Stand Age (Years) | 0–10 | 11–54 | 88–141 | 150–276 | Mean | |
|---|---|---|---|---|---|---|---|
| Statistics | |||||||
| SR | mean | 1.77 a | 4.65 b | 2.83 c | 5.18 b | 3.16 | |
| SD | 1.18 | 2.73 | 1.89 | 2.7 | |||
| CV | 66.7 | 58.7 | 66.8 | 52.1 | |||
| BR | mean | 67.38 a | 134.99 b | 151.87 b | 41.3 a | 98.89 | |
| SD | 51.89 | 99.09 | 140.52 | 48.29 | |||
| CV | 77.0 | 73.4 | 92.5 | 116.9 | |||
| SIR | mean | 245.39 a | 262.51 a | 465.86 a | 354.63 a | 332.10 | |
| SD | 177.07 | 126.92 | 318.98 | 290.65 | |||
| CV | 72.2 | 48.3 | 68.5 | 82.0 | |||
| SWC5 over the growing season | mean | 53.62 a | 39.90 b | 73.02 c | 39.41 b | 51.49 | |
| SD | 13.8 | 12.07 | 17.54 | 17.19 | |||
| CV | 25.7 | 30.3 | 24.0 | 43.6 | |||
| ST5 over the growing season | mean | 11.69 a | 8.15 b | 7.53 b | 9.69 c | 9.27 | |
| SD | 5.2 | 4.86 | 4.77 | 4.3 | |||
| CV | 44.5 | 59.6 | 63.3 | 44.4 | |||
| LitD | mean | 7.54 a | 2.84 b | 6.46 a | 5.29 a | 5.53 | |
| SD | 6.76 | 2.57 | 3.91 | 3.37 | |||
| CV | 89.7 | 90.5 | 60.5 | 63.7 | |||
| C | mean | 2.52 a | 3.82 a | 4.35 a | 4.06 a | 3.69 | |
| SD | 0.88 | 2.81 | 2.96 | 2.07 | |||
| CV | 34.9 | 73.6 | 68.0 | 51.0 | |||
| N | mean | 0.11 a | 0.175 a | 0.18 a | 0.184 a | 0.16 | |
| SD | 0.037 | 0.102 | 0.102 | 0.089 | |||
| CV | 33.6 | 58.3 | 56.7 | 48.4 | |||
| C/N | mean | 18.83 a | 17.175 a | 18.7 a | 19.94 a | 18.66 | |
| SD | 1.57 | 4.05 | 4.01 | 3.28 | |||
| CV | 8.3 | 23.6 | 21.4 | 16.4 | |||
| roots | mean | 0.23 a | 1.65 b | 3.15 c | 3.18 b | 2.05 | |
| SD | 0.57 | 1.18 | 2.69 | 6.25 | |||
| CV | 247.8 | 71.5 | 85.4 | 196.5 | |||
| VegH | mean | 2.36 a | 2.83 a | 5.49b | 2.47 a | 3.29 | |
| SD | 1.3 | 2.83 | 2.98 | 1.83 | |||
| CV | 55.1 | 100.0 | 54.3 | 74.1 | |||
| ST5 in July | mean | 12.84 a | 10.99 a | 11.54 a | 8.01 b | 10.85 | |
| SD | 3.39 | 4.92 | 4.13 | 4.27 | |||
| CV | 26.4 | 44.8 | 35.8 | 53.3 | |||
| ST5 in August | mean | 12.01 a | 7.68 b | 6.16 c | 10.36 d | 9.05 | |
| SD | 4.99 | 3.38 | 3.97 | 4.14 | |||
| CV | 41.5 | 44.0 | 64.4 | 40.0 | |||
| ST5 in September | mean | 0.63 a | 4.1 b | 1.13 a | 1 a | 1.72 | |
| SD | 0.19 | 1.29 | 0.6 | 0.45 | |||
| CV | 30.2 | 31.5 | 53.1 | 45.0 | |||
| Parameter | Kruskal-Wallis Chi-Square | |
|---|---|---|
| Age Group of the Burnt Areas | Month of the Growing Season | |
| ST5 (°C) | 63.47 * | 80.34 * |
| SWC5 (%) | 152.91 * | 38.67 * |
| Total soil C at a 0–5 cm horizon (%) | 0.93 | NA |
| Total soil N at a 0–5 cm horizon (%) | 1.45 | NA |
| C/N at a 0-5 cm soil horizon | 3.56 | NA |
| SR (µmol CO2 m−2 s−1) | 237.30 * | 73.24 * |
| Microbial BR in the 0–5 cm mineral soil horizon (mg CO2-C kg−1 day−1) | 22.01 * | NA |
| Microbial SIR in the 0–5 cm mineral soil horizon (mg CO2-C kg−1 day−1) | 4.64 | NA |
| Root biomass (roots, g) | 21.88 * | NA |
| Vegetation cover height (VegH, cm) | 66.13 * | 17.66 * |
| Litter thickness (LitD, cm) | 65.23 * | 57.66 * |
| Variables | Contribution to PC | |||
|---|---|---|---|---|
| Dim 1 | Dim 2 | Dim 3 | Dim 4 | |
| Mean SR | 3.75 | 22.92 | 2.18 | 5.28 |
| Mean summer ST5 | 16.44 | 0.08 | 4.14 | 51.62 |
| Mean summer SWC5 | 1.84 | 27.47 | 0.27 | 0.93 |
| Mean soil C content | 17.24 | 0.74 | 1.23 | 5.55 |
| Mean soil N content | 15.04 | 4.47 | 1.22 | 8.02 |
| C/N | 0.18 | 0.92 | 42.72 | 21.54 |
| Mean soil BR | 6.85 | 2.82 | 23.65 | 0.19 |
| Mean soil SIR | 11.76 | 6.85 | 5.80 | 3.44 |
| roots | 13.86 | 0.21 | 10.25 | 3.40 |
| VegH | 10.16 | 13.06 | 0.73 | 0.01 |
| LitD | 2.89 | 20.46 | 7.82 | 0.03 |
| 0–10-Year-Old Group | ||||||||||||
| Age | SR | ST5 | SWC5 | C | N | C/N | BR | SIR | roots | VegH | LitD | |
| Age | p < 0.01 | p < 0.01 | p = 0.054 | p < 0.01 | ||||||||
| SR | 0.31 | p < 0.05 | p < 0.05 | |||||||||
| ST5 | 0.32 | −0.04 | p < 0.05 | p < 0.01 | ||||||||
| SWC5 | −0.44 | 0.04 | −0.55 | p = 0.085 | ||||||||
| C | NA | NA | 0.09 | 0.99 | ||||||||
| N | NA | NA | 0.18 | 0.92 | 0.96 | |||||||
| C/N | NA | NA | 0.94 | 0.54 | 0.43 | 0.17 | ||||||
| BR | −0.16 | −0.54 | 0.73 | −0.06 | −0.23 | −0.49 | 0.78 | p < 0.01 | ||||
| SIR | NA | −0.69 | 0.52 | 0.47 | NA | NA | NA | 0.96 | ||||
| roots | NA | 0.14 | −0.53 | 0.69 | NA | NA | NA | NA | NA | |||
| VegH | 0.45 | 0.47 | NA | NA | NA | NA | NA | NA | NA | NA | ||
| LitD | −0.78 | −0.23 | −0.74 | 0.60 | NA | NA | NA | NA | NA | 0.50 | 0.33 | |
| 11–54-Year-Old Group | ||||||||||||
| Age | SR | ST5 | SWC5 | C | N | C/N | BR | SIR | roots | VegH | LitD | |
| Age | p < 0.01 | p < 0.05 | p < 0.01 | p < 0.01 | ||||||||
| SR | −0.29 | p < 0.01 | p < 0.05 | p < 0.05 | ||||||||
| ST5 | −0.07 | 0.35 | p = 0.056 | p < 0.01 | ||||||||
| SWC5 | −0.21 | 0.01 | −0.21 | p < 0.01 | ||||||||
| C | −0.11 | 0.08 | 0.43 | 0.004 | p < 0.01 | p < 0.05 | p < 0.05 | |||||
| N | −0.11 | 0.17 | 0.43 | 0.08 | 0.99 | p < 0.01 | p < 0.05 | |||||
| C/N | 0.28 | −0.21 | 0.20 | 0.11 | 0.87 | 0.84 | ||||||
| BR | −0.12 | −0.04 | 0.38 | 0.19 | 0.76 | 0.78 | 0.64 | p < 0.05 | ||||
| SIR | 0.60 | 0.59 | 0.44 | 0.21 | NA | NA | NA | 0.34 | p = 0.058 | |||
| roots | −0.24 | 0.23 | 0.64 | 0.22 | NA | NA | NA | NA | NA | |||
| VegH | 0.64 | −0.44 | −0.09 | 0.74 | NA | NA | NA | NA | NA | −0.10 | p < 0.01 | |
| LitD | 0.82 | −0.14 | −0.52 | −0.14 | NA | NA | NA | 0.87 | 0.80 | −0.11 | 0.59 | |
| 88–141-Year-Old Group | ||||||||||||
| Age | SR | ST5 | SWC5 | C | N | C/N | BR | SIR | roots | VegH | LitD | |
| Age | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.05 | ||||||
| SR | 0.17 | p < 0.05 | p < 0.01 | p < 0.01 | p < 0.01 | |||||||
| ST5 | −0.49 | 0.15 | p < 0.01 | |||||||||
| SWC5 | −0.38 | −0.28 | 0.3 | p = 0.064 | p < 0.05 | |||||||
| C | NA | −0.02 | 0.14 | 0.25 | p < 0.01 | p < 0.01 | p = 0.073 | |||||
| N | NA | −0.05 | 0.16 | 0.28 | 0.99 | p < 0.01 | p = 0.078 | |||||
| C/N | NA | −0.05 | 0.08 | 0.26 | 0.99 | 0.99 | ||||||
| BR | −0.17 | −0.21 | 0.4 | 0.27 | 0.84 | 0.84 | 0.79 | p < 0.01 | ||||
| SIR | 0.15 | −0.25 | −0.39 | 0.55 | NA | NA | NA | 0.76 | ||||
| roots | −0.50 | 0.10 | 0.16 | 0.15 | NA | NA | NA | NA | NA | |||
| VegH | −0.27 | −0.22 | 0.36 | 0.11 | NA | NA | NA | NA | NA | −0.31 | p < 0.01 | |
| LitD | 0.12 | 0.39 | 0.58 | 0.09 | NA | NA | NA | NA | NA | 0.09 | −0.15 | |
| 150–276-Year-Old Group | ||||||||||||
| Age | SR | ST5 | SWC5 | C | N | C/N | BR | SIR | roots | VegH | LitD | |
| Age | p < 0.01 | p < 0.01 | p < 0.01 | p = 0.063 | p < 0.01 | p = 0.052 | ||||||
| SR | −0.27 | p < 0.05 | p < 0.05 | p < 0.05 | p = 0.06 | p < 0.05 | ||||||
| ST5 | 0.04 | 0.08 | p = 0.07 | p < 0.05 | ||||||||
| SWC5 | −0.004 | −0.2 | −0.29 | |||||||||
| C | −0.79 | 0.64 | −0.13 | 0.29 | p < 0.01 | p = 0.085 | p = 0.061 | p < 0.05 | ||||
| N | −0.76 | 0.70 | −0.02 | 0.25 | 0.99 | p = 0.055 | p = 0.073 | p = 0.079 | p < 0.05 | |||
| C/N | -0.43 | -0.09 | -0.39 | -0.13 | 0.42 | 0.38 | ||||||
| BR | 0.42 | −0.17 | 0.50 | −0.03 | −0.50 | −0.51 | −0.46 | |||||
| SIR | −0.60 | 0.89 | 0.65 | −0.17 | 0.79 | 0.76 | 0.03 | −0.44 | ||||
| roots | 0.27 | 0.44 | 0.15 | −0.005 | NA | NA | NA | NA | NA | |||
| VegH | 0.68 | −0.22 | 0.11 | 0.005 | NA | NA | NA | NA | NA | NA | ||
| LitD | −0.26 | 0.29 | −0.05 | 0.21 | −0.89 | −0.89 | −0.5 | −0.03 | −0.83 | 0.33 | 0.16 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masyagina, O.V.; Evgrafova, S.Y.; Menyailo, O.V.; Mori, S.; Koike, T.; Prokushkin, S.G. Age-Dependent Changes in Soil Respiration and Associated Parameters in Siberian Permafrost Larch Stands Affected by Wildfire. Forests 2021, 12, 107. https://0-doi-org.brum.beds.ac.uk/10.3390/f12010107
Masyagina OV, Evgrafova SY, Menyailo OV, Mori S, Koike T, Prokushkin SG. Age-Dependent Changes in Soil Respiration and Associated Parameters in Siberian Permafrost Larch Stands Affected by Wildfire. Forests. 2021; 12(1):107. https://0-doi-org.brum.beds.ac.uk/10.3390/f12010107
Chicago/Turabian StyleMasyagina, Oxana V., Svetlana Y. Evgrafova, Oleg V. Menyailo, Shigeta Mori, Takayoshi Koike, and Stanislav G. Prokushkin. 2021. "Age-Dependent Changes in Soil Respiration and Associated Parameters in Siberian Permafrost Larch Stands Affected by Wildfire" Forests 12, no. 1: 107. https://0-doi-org.brum.beds.ac.uk/10.3390/f12010107