Carbon Emission and Redistribution among Forest Carbon Pools, and Change in Soil Nutrient Content after Different Severities of Forest Fires in Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Measurements
2.3. Laboratory Analysis
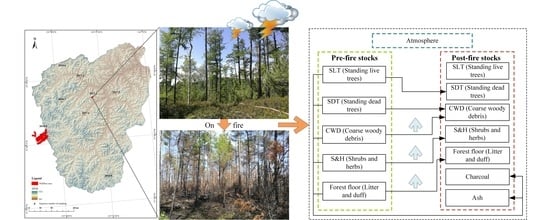
2.4. Carbon Stock Calculations
2.5. Carbon Emissions and Redistribution Estimations
2.6. Statistical Analyses
3. Results
3.1. Carbon Distribution among Various Forest Carbon Pools in Unburned and Burned Plots
3.2. The Impacts of Fire Severity on the Carbon Distribution among Various Forest Carbon Pools
3.3. Changes in Soil Nutrient and BC Contents and TOCS Shortly after Low-, Moderate-, and High-Severity Fires
3.4. Carbon Transfers among the Aboveground Carbon Pools and Emissions under Low-, Moderate-, and High-Severity Fires
4. Discussion
4.1. Carbon Distribution in Various Carbon Pools and Redistribution Following Low-, Moderate-, and High-Severity Fires
4.2. Impacts of Forest Fire Severities on Soil Nutrient and BC Contents and TOCS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.Z.; Cai, W.H.; Yang, J.; White, M.; Lhotka, J.M. Dynamics of Postfire Aboveground Carbon in a Chronosequence of Chinese Boreal Larch Forests. J. Geophys. Res. Biogeosciences 2018, 123, 3490–3506. [Google Scholar] [CrossRef]
- Wang, C.; Gower, S.T.; Wang, Y.; Zhao, H.; Yan, P.; Bond-Lamberty, B.P. The influence of fire on carbon distribution and net primary production of boreal Larix gmelinii forests in north-eastern China. Glob. Change Biol. 2001, 7, 719–730. [Google Scholar] [CrossRef]
- Kasischke, E.S. Boreal ecosystems in the global carbon cycle. In Fire, Climate Change, and Carbon Cycling in the Boreal Forest; Kasischke, E.S., Stocks, B.J., Eds.; Springer: New York, NY, USA, 2000; Volume 138. [Google Scholar]
- Seedre, M.; Shrestha, B.M.; Chen, H.Y.H.; Colombo, S.; Jõgiste, K. Carbon dynamics of North American boreal forest after stand replacing wildfire and clearcut logging. J. For. Res. 2011, 16, 168–183. [Google Scholar] [CrossRef]
- Shorohova, E.; Kuuluvainen, T.; Kangur, A.; Jõgiste, K. Natural stand structures, disturbance regimes and successional dynamics in the Eurasian boreal forests: A review with special reference to Russian studies. Ann. For. Sci. 2009, 66, 201. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R.; Chipman, M.L.; Higuera, P.E.; Stefanova, I.; Brubaker, L.B.; Hu, F.S. Recent burning of boreal forests exceeds fire regime limits of the past 10,000 years. Proc. Natl. Acad. Sci. USA 2013, 110, 13055–13060. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; He, H.S.; Hu, Y.; Bu, R.; Li, X. Historic and current fire regimes in the Great Xing’an Mountains, northeastern China: Implications for long-term forest management. For. Ecol. Manag. 2008, 254, 445–453. [Google Scholar] [CrossRef]
- Spracklen, D.V.; Mickley, L.J.; Logan, J.A.; Hudman, R.C.; Yevich, R.; Flannigan, M.D.; Westerling, A.L. Impacts of climate change from 2000 to 2050 on wildfire activity and carbonaceous aerosol concentrations in the western United States. J. Geophys. Res. Atmos. 2009, 114. [Google Scholar] [CrossRef]
- Yocom Kent, L.L.; Shive, K.L.; Strom, B.A.; Sieg, C.H.; Hunter, M.E.; Stevens-Rumann, C.S.; Fulé, P.Z. Interactions of fuel treatments, wildfire severity, and carbon dynamics in dry conifer forests. For. Ecol. Manag. 2015, 349, 66–72. [Google Scholar] [CrossRef]
- Yatskov, M.A.; Harmon, M.E.; Barrett, T.M.; Dobelbower, K.R. Carbon pools and biomass stores in the forests of Coastal Alaska: Uncertainty of estimates and impact of disturbance. For. Ecol. Manag. 2019, 434, 303–317. [Google Scholar] [CrossRef]
- Matchett, J.R.; Lutz, J.A.; Tarnay, L.W.; Smith, D.G.; Becker, K.M.L.; Brooks, M.L. Impacts of Fire Management on aboveground Tree Carbon Stocks in Yosemite and Sequoia & Kings Canyon National Parks; National Park Service: Washington, DC, USA, 2015. [Google Scholar]
- Eskelson, B.N.I.; Monleon, V.J.; Fried, J.S. A 6 year longitudinal study of post-fire woody carbon dynamics in California’s forests. Can. J. For. Res. 2016, 46, 610–620. [Google Scholar] [CrossRef]
- Chang, Y.; Zhu, Z.; Feng, Y.; Li, Y.; Bu, R.; Hu, Y. The spatial variation in forest burn severity in Heilongjiang Province, China. Nat. Hazards 2016, 81, 981–1001. [Google Scholar] [CrossRef]
- Meigs, G.W.; Donato, D.C.; Campbell, J.L.; Martin, J.G.; Law, B.E. Forest Fire Impacts on Carbon Uptake, Storage, and Emission: The Role of Burn Severity in the Eastern Cascades, Oregon. Ecosystems 2009, 12, 1246–1267. [Google Scholar] [CrossRef]
- Liu, S.; Bond-Lamberty, B.; Hicke, J.A.; Vargas, R.; Zhao, S.; Chen, J.; Edburg, S.L.; Hu, Y.; Liu, J.; McGuire, A.D.; et al. Simulating the impacts of disturbances on forest carbon cycling in North America: Processes, data, models, and challenges. J. Geophys. Res. Biogeosciences 2011, 116, G00K08. [Google Scholar] [CrossRef] [Green Version]
- Blais, A.; Lorrain, S.; Plourde, Y.; Varfalvy, L. Organic carbon densities of soils and vegetation of tropical, temperate and boreal forests. In Greenhouse Gas Emissions-Fluxes and Processes; Tremblay, A., Varfalvy, L., Roehm, C., Garneau, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar] [CrossRef]
- Wang, X.; Chang, Y.; Chen, H.; Hu, Y.; Feng, Y.; Wu, W. Biomass allocation characteristics of the main forest ecosystems in the Great Xing’an Mountains, Heilongjiang Province. Chin. J. Ecol. 2014, 33, 1437–1444. [Google Scholar]
- Amiro, B.D.; Cantin, A.C.; Flannigan, M.D.; de Groot, W.J. Future emissions from Canadian boreal forest fires. Can. J. For. Res. 2009, 39, 383–395. [Google Scholar] [CrossRef]
- Boby, L.A.; Schuur, E.A.G.; Mack, M.C.; Verbyla, D.; Johnstone, J.F. Quantifying fire severity, carbon, and nitrogen emissions in Alaska’s boreal forest. Ecol. Appl. 2010, 20, 1633–1647. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Wei, S.; Sun, L. Estimating carbon emissions from forest fires during 2001 to 2010 in Daxing’anling Mountain. Acta Ecol. Sin. 2012, 32, 5373–5386. [Google Scholar]
- Ottmar, R.D. Wildland fire emissions, carbon, and climate: Modeling fuel consumption. For. Ecol. Manag. 2014, 317, 41–50. [Google Scholar] [CrossRef]
- Angers, V.A.; Gauthier, S.; Drapeau, P.; Jayen, K.; Bergeron, Y. Tree mortality and snag dynamics in North American boreal tree species after a wildfire: A long-term study. Int. J. Wildland Fire 2011, 20, 751–763. [Google Scholar] [CrossRef]
- Carvalho, E.O.; Kobziar, L.N.; Putz, F.E. Fire ignition patterns affect production of charcoal in southern forests. Int. J. Wildland Fire 2011, 20, 474–477. [Google Scholar] [CrossRef]
- Preston, C.M.; Schmidt, M.W.I. Black (pyrogenic) carbon: A synthesis of current knowledge and uncertainties with special consideration of boreal regions. Biogeosciences 2006, 3, 397–420. [Google Scholar] [CrossRef] [Green Version]
- Bird, M.I.; Wynn, J.G.; Saiz, G.; Wurster, C.M.; McBeath, A. The Pyrogenic Carbon Cycle. Annu. Rev. Earth Planet. Sci. 2015, 43, 273–298. [Google Scholar] [CrossRef]
- Maestrini, B.; Alvey, E.; Hurteau, M.; Safford, H.; Miesel, J. Fire severity alters the distribution of pyrogenic carbon stocks across ecosystem pools in a Californian mixed-conifer forest. J. Geophys. Res. Biogeosciences 2017, 122, 2338–2355. [Google Scholar] [CrossRef]
- Santín, C.; Doerr, S.H.; Kane, E.S.; Masiello, C.A.; Ohlson, M.; de la Rosa, J.M.; Preston, C.M.; Dittmar, T. Towards a global assessment of pyrogenic carbon from vegetation fires. Glob. Change Biol. 2016, 22, 76–91. [Google Scholar] [CrossRef]
- Huang, W.; Hu, Y.; Chang, Y.; Liu, M.; Li, Y.; Ren, B.; Shi, S. Effects of Fire Severity and Topography on Soil Black Carbon Accumulation in Boreal Forest of Northeast China. Forests 2018, 9, 408. [Google Scholar] [CrossRef] [Green Version]
- Smithwick, E.A.H.; Turner, M.G.; Mack, M.C.; Chapin, F.S. Postfire Soil N Cycling in Northern Conifer Forests Affected by Severe, Stand-Replacing Wildfires. Ecosystems 2005, 8, 163–181. [Google Scholar] [CrossRef]
- González-Pérez, J.A.; González-Vila, F.J.; Almendros, G.; Knicker, H. The effect of fire on soil organic matter—A review. Environ. Int. 2004, 30, 855–870. [Google Scholar] [CrossRef]
- Hu, T.; Hu, H.; Li, F.; Zhao, B.; Wu, S.; Zhu, G.; Sun, L. Long-term effects of post-fire restoration types on nitrogen mineralisation in a Dahurian larch (Larix gmelinii) forest in boreal China. Sci. Total Environ. 2019, 679, 237–247. [Google Scholar] [CrossRef]
- Sun, M. The Impact on Soil Properties and Revegetation from Forest Fire in Tahe forest Region; Beijing Forestry University: Beijing, China, 2011. [Google Scholar]
- Gu, H.; Jin, Y.; Zhang, Y.; Chen, X. Effects of forest fire on soil nutrients of Ass. Pinus pumila-Larix gmelinii forest in Great Xing’an Mountains. J. Beijing For. 2016, 38, 48–54. [Google Scholar] [CrossRef]
- Francos, M.; Pereira, P.; Mataix-Solera, J.; Arcenegui, V.; Alcañiz, M.; Ubeda, X. How clear-cutting affects fire severity and soil properties in a Mediterranean ecosystem. J. Environ. Manag. 2018, 206, 625–632. [Google Scholar] [CrossRef]
- Butnor, J.R.; Johnsen, K.H.; Maier, C.A.; Nelson, C.D. Intra-Annual Variation in Soil C, N and Nutrients Pools after Prescribed Fire in a Mississippi Longleaf Pine (Pinus palustris Mill.) Plantation. Forests 2020, 11, 181. [Google Scholar] [CrossRef] [Green Version]
- Lavoie, M.; Starr, G.; Mack, M.; Martin, T.; Gholz, H. Effects of a Prescribed Fire on Understory Vegetation, Carbon Pools, and Soil Nutrients in a Longleaf Pine-Slash Pine Forest in Florida. Nat. Areas J. 2010, 30, 82–94. [Google Scholar] [CrossRef]
- Hu, M.; Liu, Y.; Wang, T.; Hao, Y.; Li, Z.; Wan, S. Fire Alters Soil Properties and Vegetation in a Coniferous–Broadleaf Mixed Forest in Central China. Forests 2020, 11, 164. [Google Scholar] [CrossRef] [Green Version]
- Schoenholtz, S.H.; Miegroet, H.V.; Burger, J.A. A review of chemical and physical properties as indicators of forest soil quality: Challenges and opportunities. For. Ecol. Manag. 2000, 138, 335–356. [Google Scholar] [CrossRef]
- Yu, G.; Li, X.; Wang, Q.; Li, S. Carbon Storage and Its Spatial Pattern of Terrestrial Ecosystem in China. J. Resour. Ecol. 2010, 1, 97–109, 113. [Google Scholar]
- Jia, Y.; Chang, Y.; Ping, X.; Chang, C. Dynamics of carbon stocks of different pools in Huzhong National Natural Reserve, Northeast China under the disturbance of various severity fires. Chin. J. Appl. Ecol. 2021, 32, 2325–2334. [Google Scholar]
- Harden, J.W.; Trumbore, S.E.; Stocks, B.J.; Hirsch, A.; Gower, S.T.; O’Neill, K.P.; Kasischke, E.S. The role of fire in the boreal carbon budget. Glob. Change Biol. 2000, 6, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, W.; Chang, Y.; Feng, Y.; Chen, H.; Hu, Y.; Chi, J. Fire severity of burnt area in Huzhong forest region of Great Xing’an Mountains, Northeast China based on normalized burn ratio analysis. Chin. J. Appl. Ecol. 2013, 24, 967–974. [Google Scholar]
- Ping, X.; Chang, Y.; Liu, M.; Hu, Y.; Yuan, Z.; Shi, S.; Jia, Y.; Li, D.; Yu, L. Fuel burning efficiency under various fire severities of a boreal forest landscape in north-east China. Int. J. Wildland Fire 2021, 30, 691–701. [Google Scholar] [CrossRef]
- Lu, R. Analytical Methods of Soil Agricultural Chemistry; China Agricultural Science Press: Beijing, China, 1999. [Google Scholar]
- Cheng, Y.X.; Li, Z.X. A study on biomass of three main forest types in Larix gmelinii forest. Inn. Mong. For. Investig. Des. 1989, 4, 89–100. [Google Scholar]
- Chen, C.G.; Zhu, J.F. A Handbook of Biomass of Main Tree Species in the Northeastern China; China Forestry Press: Beijing, China, 1989. [Google Scholar]
- Luo, T.X. Patterns of Net Primary Productivity for Chinese Major Forest Types and Their Mathematical Models; Graduate University of Chinese Academy of Sciences: Beijing, China, 1996. [Google Scholar]
- Kang, L. A Study on the Decomposition of Falled Trees of Larix Gmelinii and Betula Platyphylla Forests in Daxinganling Mountains; Inner Mongolia Agricultural Universtiy: Hohhot, China, 2012. [Google Scholar]
- Waddell, K.L. Sampling coarse woody debris for multiple attributes in extensive resource inventories. Ecol. Indic. 2002, 1, 139–153. [Google Scholar] [CrossRef]
- Hu, H.Q.; Sun, L.; Guo, Q.X.; Lü, X.S. Carbon emissions from forest fires on main Arbor species in Daxing’an Mountains in Heilongjiang province. Sci. Silvae Sin. 2007, 43, 82–88. [Google Scholar] [CrossRef]
- Seiler, W.; Crutzen, P.J. Estimates of gross and net fluxes of carbon between the biosphere and the atmosphere from biomass burning. Clim. Change 1980, 2, 207–247. [Google Scholar] [CrossRef]
- French, N.H.F.; de Groot, W.J.; Jenkins, L.K.; Rogers, B.M.; Alvarado, E.; Amiro, B.; de Jong, B.; Goetz, S.; Hoy, E.; Hyer, E.; et al. Model comparisons for estimating carbon emissions from North American wildland fire. J. Geophys. Res. 2011, 116, G00K05. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 4 March 2020).
- Galarza-Macias, J. Incorporating Fire Severity into Estimates of Carbon Losses in Ontario’s Boreal Forest; Trent University: Peterborough, ON, Canada, 2010. [Google Scholar]
- Melvin, A.M.; Mack, M.C.; Johnstone, J.F.; David McGuire, A.; Genet, H.; Schuur, E.A.G. Differences in Ecosystem Carbon Distribution and Nutrient Cycling Linked to Forest Tree Species Composition in a Mid-Successional Boreal Forest. Ecosystems 2015, 18, 1472–1488. [Google Scholar] [CrossRef]
- Litton, C.M.; Raich, J.W.; Ryan, M.G. Carbon allocation in forest ecosystems. Glob. Change Biol. 2007, 13, 2089–2109. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhang, J.; Han, A.; Wang, X.; Wang, X. Biomass and carbon pool of Larix gmelini young and middle age forest in Xing’an Mountains Inner Mongolia. Acta Ecol. Sin. 2007, 27, 1756–1762. [Google Scholar]
- Hu, H.; Luo, B.; Wei, S.; Wei, S.; Wen, Z.; Sun, L.; Luo, S.; Wang, L.; Ma, H. Estimating biological carbon storage of five typical forest types in the Daxinganling Mountains, Heilongjiang, China. Acta Ecol. Sin. 2015, 35, 5745–5760. [Google Scholar]
- Dyck, B.S.; Shay, J.M. Biomass and carbon pool of two bogs in the Experimental Lakes Area, northwestern Ontario. Can. J. Bot.-Rev. Can. Bot. 1999, 77, 291–304. [Google Scholar] [CrossRef]
- Turner, M.G.; Romme, W.H. Landscape dynamics in crown fire ecosystems. Landsc. Ecol. 1994, 9, 59–77. [Google Scholar] [CrossRef]
- van Bellen, S.; Garneau, M.; Bergeron, Y. Impact of Climate Change on Forest Fire Severity and Consequences for Carbon Stocks in Boreal Forest Stands of Quebec, Canada: A Synthesis. Fire Ecol. 2010, 6, 16–44. [Google Scholar] [CrossRef]
- Carlson, C.H.; Dobrowski, S.Z.; Safford, H.D. Variation in tree mortality and regeneration affect forest carbon recovery following fuel treatments and wildfire in the Lake Tahoe Basin, California, USA. Carbon Balance Manag. 2012, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Stocks, B.J.; Kauffman, J.B. Biomass Consumption and Behavior of Wildland Fires in Boreal, Temperate, and Tropical Ecosystems: Parameters Necessary to Interpret Historic Fire Regimes and Future Fire Scenarios; Clark, J.S., Cachier, H., Goldammer, J.G., Stocks, B., Eds.; Sediment Records of Biomass Burning and Global Change; Springer: Berlin/Heidelberg, Germany, 1997; pp. 169–188. [Google Scholar]
- Righi, C.A.; de Alencastro Graça, P.M.L.; Cerri, C.C.; Feigl, B.J.; Fearnside, P.M. Biomass burning in Brazil’s Amazonian “arc of deforestation”: Burning efficiency and charcoal formation in a fire after mechanized clearing at Feliz Natal, Mato Grosso. For. Ecol. Manag. 2009, 258, 2535–2546. [Google Scholar] [CrossRef]
- Czimczik, C.I.; Schmidt, M.W.I.; Schulze, E.-D. Effects of increasing fire frequency on black carbon and organic matter in Podzols of Siberian Scots pine forests. Eur. J. Soil Sci. 2005, 56, 417–428. [Google Scholar] [CrossRef] [Green Version]
- Krishnaraj, S.J.; Baker, T.G.; Polglase, P.J.; Volkova, L.; Weston, C.J. Prescribed fire increases pyrogenic carbon in litter and surface soil in lowland Eucalyptus forests of south-eastern Australia. For. Ecol. Manag. 2016, 366, 98–105. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Zhao, P. Post-fire dispersal characteristics of charcoal particles in the Daxing’an Mountains of north-east China and their implications for reconstructing past fire activities. Int. J. Wildland Fire 2017, 26, 46–57. [Google Scholar] [CrossRef]
- Umbanhowar, C.E.; McGrath, M.J. Experimental production and analysis of microscopic charcoal from wood, leaves and grasses. Holocene 1998, 8, 341–346. [Google Scholar] [CrossRef]
- Francos, M.; Úbeda, X.; Pereira, P.; Alcañiz, M. Long-term impact of wildfire on soils exposed to different fire severities. A case study in Cadiretes Massif (NE Iberian Peninsula). Sci. Total Environ. 2018, 615, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Rutigliano, F.A.; Fierro, A.R.; De Pascale, R.A.; De Marco, A.; Virzo De Santo, A. Role of fire on soil organic matter turnover and microbial activity in a mediterranean burned area. In Developments in Soil Science; Violante, A., Huang, P.M., Bollag, J.M., Gianfreda, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 28, pp. 205–215. [Google Scholar]
- Schafer, J.; Mack, M. Short-term effects of fire on soil and plant nutrients in palmetto flatwoods. Plant Soil 2010, 334, 433–447. [Google Scholar] [CrossRef]
- Raison, R.J.; Khanna, P.K.; Woods, P.V. Mechanisms of element transfer to the atmosphere during vegetation fires. Can. J. For. Res. 1985, 15, 132–140. [Google Scholar] [CrossRef]
- Ficken, C.; Wright, J. Contributions of microbial activity and ash deposition to post-fire nitrogen availability in a pine savanna. Biogeosciences 2017, 14, 241–255. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M. The Effect of Forest Fire on Soil Environment; Northeast Forestry University: Harbin, China, 2002. [Google Scholar]
- Roy, D.P.; Boschetti, L.; Maier, S.W.; Smith, A.M.S. Field estimation of ash and char colour-lightness using a standard grey scale. Int. J. Wildland Fire 2010, 19, 698–704. [Google Scholar] [CrossRef]







| Site | Date of Fire Occurrence (Day, Month, Year) | Date of Field Investigation (Day, Month, Year) | Investigation Time since Fire (Days) |
|---|---|---|---|
| 2017_1 | 23,06,2017 | 28,07,2017 | 35 |
| 2017_2 | 24,06,2017 | 29,07,2017 | 35 |
| 2017_3 | 25,06,2017 | 30,07,2017 | 35 |
| 2017_4 | 03,07,2017 | 30,07,2017 | 28 |
| 2018_5 | 03,06,2018 | 25,07,2018 | 52 |
| 2018_6 | 31,05,2018 | 29,07,2018 | 59 |
| 2018_7 | 01,06,2018 | 30,07,2018 | 59 |
| 2018_8 | 31,05,2018 | 01,08,2018 | 62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ping, X.; Chang, Y.; Liu, M.; Hu, Y.; Huang, W.; Shi, S.; Jia, Y.; Li, D. Carbon Emission and Redistribution among Forest Carbon Pools, and Change in Soil Nutrient Content after Different Severities of Forest Fires in Northeast China. Forests 2022, 13, 110. https://0-doi-org.brum.beds.ac.uk/10.3390/f13010110
Ping X, Chang Y, Liu M, Hu Y, Huang W, Shi S, Jia Y, Li D. Carbon Emission and Redistribution among Forest Carbon Pools, and Change in Soil Nutrient Content after Different Severities of Forest Fires in Northeast China. Forests. 2022; 13(1):110. https://0-doi-org.brum.beds.ac.uk/10.3390/f13010110
Chicago/Turabian StylePing, Xiaoying, Yu Chang, Miao Liu, Yuanman Hu, Wentao Huang, Sixue Shi, Yuchen Jia, and Dikang Li. 2022. "Carbon Emission and Redistribution among Forest Carbon Pools, and Change in Soil Nutrient Content after Different Severities of Forest Fires in Northeast China" Forests 13, no. 1: 110. https://0-doi-org.brum.beds.ac.uk/10.3390/f13010110