Large-Scale Screening of Thiol and Fermentative Aroma Production during Wine Alcoholic Fermentation: Exploring the Effects of Assimilable Nitrogen and Peptides
Abstract
:1. Introduction
2. Materials and Methods
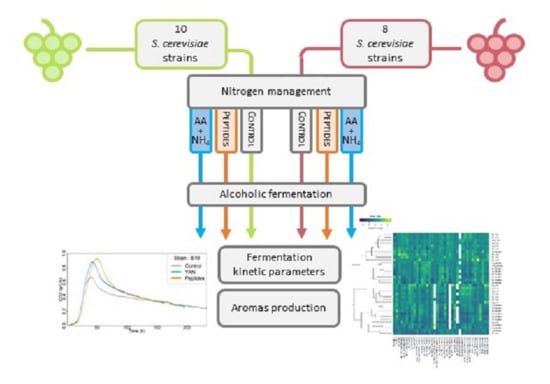
2.1. Yeast Strains
2.2. Fermentation Media
2.3. Small Peptide Preparation
2.4. Fermentation Conditions
2.5. Determination of the Parameters of Fermentation Kinetics
2.6. Sample Preparation
2.7. Determination of Metabolite Concentrations
2.8. Analysis of Fermentative Aromas by Gas Chromatography/Mass Spectrometry
2.9. Thiol Analysis
2.10. Statistical Analysis
3. Results
3.1. Effect of the Nitrogen Sources on Fermentation Kinetics
3.2. Effect of Nitrogen Sources on Yeast Fermentation Byproducts during Fermentation
3.3. Effect of Nitrogen Sources on Fermentative Aroma and Thiol Production during Fermentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tesnière, C. Importance and role of lipids in wine yeast fermentation. Appl. Microbiol. Biotechnol. 2019, 103, 8293–8300. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.; Tourdot-Maréchal, R.; Sparrow, C.; Morge, C.; Alexandre, H. Influence of nitrogen status in wine alcoholic fermentation. Food Microbiol. 2019, 83, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Duc, C.; Pradal, M.; Sanchez, I.; Noble, J.; Tesnière, C.; Blondin, B. A set of nutrient limitations trigger yeast cell death in a nitrogen-dependent manner during wine alcoholic fermentation. PLoS ONE 2017, 12, e0184838. [Google Scholar] [CrossRef] [PubMed]
- Henschke, P.A.; Jiranek, V. Yeasts—Metabolism of nitrogen compounds. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publichers GmbH: Chur, Switzerland, 1993; pp. 77–164. [Google Scholar]
- Yokotsuka, K.; Fukui, M. Changes in nitrogen compounds in berries of six grape cultivars during ripening over two years. Am. J. Enol. Vitic. 2002, 53, 69–77. [Google Scholar]
- Marsit, S.; Mena, A.; Bigey, F.; Sauvage, F.-X.; Couloux, A.; Guy, J.; Legras, J.-L.; Barrio, E.; Dequin, S.; Galeote, V. Evolutionary advantage conferred by an eukaryote-to-eukaryote gene transfer event in wine yeasts. Mol. Biol. Evol. 2015, 32, 1695–1707. [Google Scholar] [CrossRef] [Green Version]
- Homann, O.R.; Cai, H.; Becker, J.M.; Lindquist, S.L. Harnessing natural diversity to probe metabolic pathways. PLoS Genet. 2005, 1, e80. [Google Scholar] [CrossRef]
- Cai, H.; Hauser, M.; Naider, F.; Becker, J.M. Differential regulation and substrate preferences in two peptide transporters of Saccharomyces cerevisiae. Eukaryot. Cell 2007, 6, 1805–1813. [Google Scholar] [CrossRef] [Green Version]
- Damon, C.; Vallon, L.; Zimmermann, S.; Haider, M.Z.; Galeote, V.; Dequin, S.; Luis, P.; Fraissinet-Tachet, L.; Marmeisse, R. A novel fungal family of oligopeptide transporters identified by functional metatranscriptomics of soil eukaryotes. ISME J. 2011, 5, 1871–1880. [Google Scholar] [CrossRef]
- Marsit, S.; Sanchez, I.; Galeote, V.; Dequin, S. Horizontally acquired oligopeptide transporters favour adaptation of Saccharomyces cerevisiae wine yeast to oenological environment. Environ. Microbiol. 2016, 18, 1148–1161. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef]
- Rollero, S.; Bloem, A.; Camarasa, C.; Sanchez, I.; Ortiz-Julien, A.; Sablayrolles, J.-M.; Dequin, S.; Mouret, J.-R. Combined effects of nutrients and temperature on the production of fermentative aromas by Saccharomyces cerevisiae during wine fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 2291–2304. [Google Scholar] [CrossRef] [PubMed]
- Seguinot, P.; Rollero, S.; Sanchez, I.; Sablayrolles, J.-M.; Ortiz-Julien, A.; Camarasa, C.; Mouret, J.-R. Impact of the timing and the nature of nitrogen additions on the production kinetics of fermentative aromas by Saccharomyces cerevisiae during winemaking fermentation in synthetic media. Food Microbiol. 2018, 76, 29–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, C.; Mendes-Faia, A.; Mendes-Ferreira, A. The nitrogen source impacts major volatile compounds released by Saccharomyces cerevisiae during alcoholic fermentation. Int. J. Food Microbiol. 2012, 160, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.-J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Hernandez-Orte, P.; Bely, M.; Cacho, J.; Ferreira, V. Impact of ammonium additions on volatile acidity, ethanol, and aromatic compound production by different Saccharomyces cerevisiae strains during fermentation in controlled synthetic media. Aust. J. Grape Wine Res. 2006, 12, 150–160. [Google Scholar] [CrossRef]
- Jiménez-Martí, E.; Aranda, A.; Mendes-Ferreira, A.; Mendes-Faia, A.; del Olmo, M.L. The nature of the nitrogen source added to nitrogen depleted vinifications conducted by a Saccharomyces cerevisiae strain in synthetic must affects gene expression and the levels of several volatile compounds. Antonie Van Leeuwenhoek 2007, 92, 61–75. [Google Scholar] [CrossRef]
- Mestres, M.; Busto, O.; Guasch, J. Analysis of organic sulfur compounds in wine aroma. J. Chromatogr. A 2000, 881, 569–581. [Google Scholar] [CrossRef]
- Howell, K.S.; Klein, M.; Swiegers, J.H.; Hayasaka, Y.; Elsey, G.M.; Fleet, G.H.; Høj, P.B.; Pretorius, I.S.; de Barros Lopes, M.A. Genetic determinants of volatile-thiol release by Saccharomyces cerevisiae during wine fermentation. Appl. Environ. Microbiol. 2005, 71, 5420–5426. [Google Scholar] [CrossRef] [Green Version]
- Tominaga, T.; Murat, M.-L.; Dubourdieu, D. Development of a method for analyzing the volatile thiols involved in the characteristic aroma of wines made from Vitis vinifera L. Cv. Sauvignon Blanc. J. Agr. Food Chem. 1998, 46, 1044–1048. [Google Scholar] [CrossRef]
- Roland, A.; Schneider, R.; Razungles, A.; Cavelier, F. Varietal thiols in wine: Discovery, analysis and applications. Chem. Rev. 2011, 111, 7355–7376. [Google Scholar] [CrossRef]
- Bouchilloux, P.; Darriet, P.; Henry, R.; Lavigne-Cruège, V.; Dubourdieu, D. Identification of volatile and powerful odorous thiols in Bordeaux red wine varieties. J. Agr. Food Chem. 1998, 46, 3095–3099. [Google Scholar] [CrossRef]
- Subileau, M.; Schneider, R.; Salmon, J.-M.; Degryse, E. Nitrogen catabolite repression modulates the production of aromatic thiols characteristic of Sauvignon Blanc at the level of precursor transport. FEMS Yeast Res. 2008, 8, 771–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bely, M.; Sablayrolles, J.M.; Barre, P. Description of alcoholic fermentation kinetics: Its variability and significance. Am. J. Enol. Vitic. 1990, 41, 319–324. [Google Scholar]
- Scheiner, D. Determination of ammonia and Kjeldahl nitrogen by indophenol method. Water Res. 1976, 10, 31–36. [Google Scholar] [CrossRef]
- Bloem, A.; Rollero, S.; Seguinot, P.; Crepin, L.; Perez, M.; Picou, C.; Camarasa, C. Workflow based on the combination of isotopic tracer experiments to investigate microbial metabolism of multiple nutrient sources. J. Vis. Exp. 2018, 131, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Deroite, A.; Legras, J.L.; Rigou, P.; Ortiz-Julien, A.; Dequin, S. Lipids modulate acetic acid and thiol final concentrations in wine during fermentation by Saccharomyces cerevisiae × Saccharomyces kudriavzevii hybrids. AMB Express 2018, 8, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roland, A.; Delpech, S.; Dagan, L.; Ducasse, M.-A.; Cavelier, F.; Schneider, R. Innovative analysis of 3-mercaptohexan-1-ol, 3-mercaptohexylacetate and their corresponding disulfides in wine by stable isotope dilution assay and nano-liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1468, 154–163. [Google Scholar] [CrossRef]
- Capone, D.L.; Ristic, R.; Pardon, K.H.; Jeffery, D.W. Simple quantitative determination of potent thiols at ultratrace levels in wine by derivatization and high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS) Analysis. Anal. Chem. 2015, 87, 1226–1231. [Google Scholar] [CrossRef]
- Mendes-Ferreira, A.; Mendes-Faia, A.; Leao, C. Growth and fermentation patterns of Saccharomyces cerevisiae under different ammonium concentrations and its implications in winemaking industry. J. Appl. Microbiol. 2004, 97, 540–545. [Google Scholar] [CrossRef]
- Tesnière, C.; Delobel, P.; Pradal, M.; Blondin, B. Impact of nutrient imbalance on wine alcoholic fermentations: Nitrogen excess enhances yeast cell death in lipid-limited must. PLoS ONE 2013, 8, e61645. [Google Scholar] [CrossRef] [Green Version]
- Bourbouloux, A.; Shahi, P.; Chakladar, A.; Delrot, S.; Bachhawat, A.K. Hgt1p, a high affinity glutathione transporter from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2000, 275, 13259–13265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aouida, M.; Khodami-Pour, A.; Ramotar, D. Novel role for the Saccharomyces cerevisiae oligopeptide transporter Opt2 in drug detoxification. Biochem. Cell Biol. 2009, 87, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Mukai, N.; Furukawa, Y.; Adachi, K.; Mizuno, A.; Iefuji, H. Effect of soy peptide on brewing beer. J. Biosci. Bioeng. 2008, 105, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Saerens, S.M.G.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadière, A.; Ortiz-Julien, A.; Camarasa, C.; Dequin, S. Evolutionary engineered Saccharomyces cerevisiae wine yeast strains with increased in vivo flux through the pentose phosphate pathway. Metab. Eng. 2011, 13, 263–271. [Google Scholar] [CrossRef]
- Bloem, A.; Sanchez, I.; Dequin, S.; Camarasa, C. Metabolic impact of redox cofactor perturbations on the formation of aroma compounds in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2016, 82, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zhang, B.; Jiang, R. Improving acetyl-CoA biosynthesis in Saccharomyces cerevisiae via the overexpression of pantothenate kinase and PDH bypass. Biotechnol. Biofuels 2017, 10, 41. [Google Scholar] [CrossRef] [Green Version]
- Verstrepen, K.J.; Van Laere, S.D.M.; Vanderhaegen, B.M.P.; Derdelinckx, G.; Dufour, J.-P.; Pretorius, I.S.; Winderickx, J.; Thevelein, J.M.; Delvaux, F.R. Expression levels of the yeast alcohol acetyltransferase genes ATF1, Lg-ATF1, and ATF2 control the formation of a broad range of volatile esters. Appl. Environ. Microbiol. 2003, 69, 5228–5237. [Google Scholar] [CrossRef] [Green Version]
- Mouret, J.R.; Camarasa, C.; Angenieux, M.; Aguera, E.; Perez, M.; Farines, V.; Sablayrolles, J.M. Kinetic analysis and gas–liquid balances of the production of fermentative aromas during winemaking fermentations: Effect of assimilable nitrogen and temperature. Food Res. Int. 2014, 62, 1–10. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Kievit, R.L.; Siebert, T.; Lattey, K.A.; Bramley, B.R.; Francis, I.L.; King, E.S.; Pretorius, I.S. The influence of yeast on the aroma of Sauvignon Blanc wine. Food Microbiol. 2009, 26, 204–211. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duc, C.; Maçna, F.; Sanchez, I.; Galeote, V.; Delpech, S.; Silvano, A.; Mouret, J.-R. Large-Scale Screening of Thiol and Fermentative Aroma Production during Wine Alcoholic Fermentation: Exploring the Effects of Assimilable Nitrogen and Peptides. Fermentation 2020, 6, 98. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation6040098
Duc C, Maçna F, Sanchez I, Galeote V, Delpech S, Silvano A, Mouret J-R. Large-Scale Screening of Thiol and Fermentative Aroma Production during Wine Alcoholic Fermentation: Exploring the Effects of Assimilable Nitrogen and Peptides. Fermentation. 2020; 6(4):98. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation6040098
Chicago/Turabian StyleDuc, Camille, Faïza Maçna, Isabelle Sanchez, Virginie Galeote, Stéphane Delpech, Anthony Silvano, and Jean-Roch Mouret. 2020. "Large-Scale Screening of Thiol and Fermentative Aroma Production during Wine Alcoholic Fermentation: Exploring the Effects of Assimilable Nitrogen and Peptides" Fermentation 6, no. 4: 98. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation6040098