Using Isotopic Data to Evaluate Esox lucius (Linnaeus, 1758) Natal Origins in a Hydrologically Complex River Basin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Otolith Collection and Processing
2.2. Statistical Methods
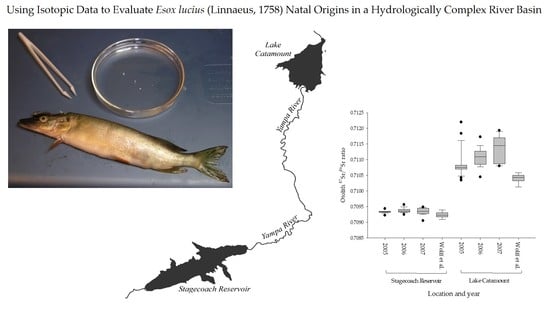
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leprieur, F.; Brosse, S.; García-Berthou, E.; Oberdorff, T.; Olden, J.D.; Townsend, C.R. Scientific uncertainty and the assessment of risks posed by non-native freshwater fishes. Fish Fish. 2009, 10, 88–97. [Google Scholar] [CrossRef]
- Vitule, J.R.S.; Freire, C.A.; Simberloff, D. Introduction of non-native freshwater fish can certainly be bad. Fish Fish. 2009, 10, 98–108. [Google Scholar] [CrossRef]
- Gallardo, B.; Clavero, M.; Sánchez, M.I.; Vilá, M. Global ecological impacts of invasive species in aquatic ecosystems. Glob. Chang. Biol. 2016, 22, 151–163. [Google Scholar] [CrossRef]
- Vander Zanden, M.J.; Lapointe, N.W.R.; Marchetti, M.P. Non-indigenous fishes and their role in freshwater imperilment. In Conservation of Freshwater Fishes; Closs, G.P., Krkosek, M., Olden, J.D., Eds.; Cambridge University Press: Cambridge, UK, 2016; Volume 1, pp. 238–269. [Google Scholar]
- Rahel, F.J.; Smith, M.A. Pathways of unauthorized fish introductions and types of management responses. Hydrobiologia 2018, 817, 41–56. [Google Scholar] [CrossRef]
- Britton, J.R.; Gozlan, R.E.; Copp, G.H. Managing non-native fish in the environment. Fish Fish. 2011, 12, 256–274. [Google Scholar] [CrossRef]
- Cucherosset, J.; Olden, J.D. Ecological impacts of non-native freshwater fishes. Fisheries 2011, 36, 215–230. [Google Scholar] [CrossRef]
- Bergman, P.K.; Haw, F.; Blankenship, H.L.; Buckley, R.M. Perspectives on design, use, and misuse of fish tags. Fisheries 1992, 17, 20–25. [Google Scholar] [CrossRef]
- Pierce, R.B.; Tomcko, C.M. Tag loss and handling mortality for northern pike marked with plastic anchor tags. N. Am. J. Fish. Manag. 1993, 13, 613–615. [Google Scholar] [CrossRef]
- Campana, S.E.; Chouinard, G.A.; Janson, J.M.; Frechet, A.; Brattey, J. Otolith elemental fingerprints as biological tracers of fish stocks. Fish. Res. 2000, 46, 343–357. [Google Scholar] [CrossRef]
- Schaffler, J.J.; Winkelman, D.L. Temporal and spatial variability in otolith trace-element signatures of juvenile striped bass from spawning locations in Lake Texoma, Oklahoma-Texas. Trans. Am. Fish. Soc. 2008, 137, 818–829. [Google Scholar] [CrossRef]
- Schaffler, J.J.; Young, S.P.; Herrington, S.; Ingram, T.; Tannehill, J. Otolith chemistry to determine within-river origins of Alabama Shad in the Apalachicola-Chattahoochee-Flint River basin. Trans. Am. Fish. Soc. 2015, 144, 1–10. [Google Scholar] [CrossRef]
- Schulz-Mirbach, T.; Ladich, F.; Plath, M.; Heß, M. Enigmatic ear stones: What we know about the functional role and evolution of fish otoliths. Biol. Rev. 2019, 94, 457–482. [Google Scholar] [CrossRef]
- Hüssy, K.; Limburg, K.E.; de Pontual, H.; Thomas, O.R.B.; Cook, P.K.; Heimbrand, Y.; Blass, M.; Sturrock, A.M. Trace element patterns in otoliths: The role of biomineralization. Rev. Fish. Sci. Aquac. 2020, 1–32. [Google Scholar] [CrossRef]
- Campana, S.E.; Neilson, J.D. Microstructure of fish otoliths. Can. J. Fish. Aquat. Sci. 1985, 42, 1014–1032. [Google Scholar] [CrossRef]
- Bestgen, K.R.; Bundy, J.M. Environmental factors affect daily increment deposition and otolith growth in young Colorado Squawfish. Trans. Am. Fish. Soc. 1998, 127, 105–117. [Google Scholar] [CrossRef]
- Haworth, M.R.; Bestgen, K.R. Daily increment validation and effects of streamflow variability and water temperature on growth of age-0 Flathead Chub. N. Am. J. Fish. Manag. 2016, 36, 744–753. [Google Scholar] [CrossRef]
- Pacheco, C.; Bustamante, C.; Araya, M. Mass-effect: Understanding the relationship between age and otolith weight in fishes. Fish Fish. 2021, 22, 623–633. [Google Scholar] [CrossRef]
- D’Iglio, C.; Albano, M.; Famulari, S.; Savoca, S.; Panarello, G.; Di Paola, D.; Perdichizzi, A.; Rinelli, P.; Lanteri, G.; Spanò, N.; et al. Intra-and interspecific variability among congeneric Pagellus otoliths. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Torres, G.J.; Lombarte, A.; Morales-Nin, B. Sagittal otolith size and shape variability to identify geographical intraspecific differences in three species of the genus Merluccius. J. Mar. Biol. Assoc. UK 2000, 80, 333–342. [Google Scholar] [CrossRef]
- Campana, S.E. Chemistry and composition of fish otoliths: Pathways, mechanisms and applications. Mar. Ecol.-Prog. Ser. 1999, 188, 263–297. [Google Scholar] [CrossRef] [Green Version]
- Carlson, K.A.; Bailey, P.E.; Fincel, M.J.; Graeb, B.D.S. Otoliths as elemental tracers of walleye environmental history: Insights for interjurisdictional fisheries management. Lake Reserv. Manag. 2016, 32, 329–340. [Google Scholar] [CrossRef]
- Carlson, K.A.; Fincel, M.J.; Graeb, B.D.S. Otolith microchemistry reveals natal origins of walleyes in Missouri River reservoirs. N. Am. J. Fish. Manag. 2016, 36, 341–350. [Google Scholar] [CrossRef]
- Avigliano, E.; Pisonero, J.; Sánchez, S.; Dománico, A.; Volpedo, A.V. Estimating contributions from nursery areas to fish stocks in freshwater systems using otolith fingerprints: The case of the streaked prochilod in the La Plata Basin (South American). River Res. Appl. 2018, 34, 863–872. [Google Scholar] [CrossRef]
- Lazartigues, A.; Girard, C.; Brodeur, P.; Lecomte, F.; Mingelbeir, M.; Sirois, P. Otolith microchemistry to identify sources of larval yellow perch in a fluvial lake: An approach towards freshwater fish management. Can. J. Fish. Aquat. Sci. 2018, 75, 474–487. [Google Scholar] [CrossRef]
- Maguffee, A.C.; Reilly, R.; Clark, R.; Jones, M.L. Examining the potential of otolith chemistry to determine natal origins of wild Lake Michigan Chinook salmon. Can. J. Fish. Aquat. Sci. 2019, 76, 2035–2044. [Google Scholar] [CrossRef]
- David, B.O.; Jarvis, M.; Özkundakci, D.; Collier, K.J.; Hicks, A.D.; Reid, M. To sea or not to sea? Multiple lines of evidence reveal the contribution of non-diadromous recruitment for supporting endemic fish populations within New Zealand’s longest river. Aquat. Conserv. 2018, 29, 1409–1423. [Google Scholar] [CrossRef]
- Arai, T.; Taha, H.; Amalina, R.; Iizuka, Y.; Chang, C. Anadromy and heterogeneous population of tropical shad Tenualosa ilisha in Malaysia, as revealed by otolith microchemistry and molecular evidence. J. Fish. Biol. 2019, 95, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.O.; Schinbeckler, R.; Viadero, N.; Larkin, M.F.; Rennert, J.J.; Shenker, J.M.; Rehage, J.S. Linking bonefish (Albula vulpes) populations to nearshore estuarine habitats using an otolith microchemistry approach. Environ. Biol. Fish. 2019, 102, 267–283. [Google Scholar] [CrossRef]
- Perrion, M.A.; Kaemingk, M.A.; Koupal, K.D.; Schoenebeck, C.W.; Bickford, N.A. Use of otolith chemistry to assess recruitment of a white bass fishery in a Nebraska reservoir. Lake Reserv. Manag. 2020, 36, 64–74. [Google Scholar] [CrossRef]
- Gibson-Reneimer, D.K.; Johnson, B.M.; Martinez, P.J.; Winkelman, D.L.; Koenig, A.E.; Woodhead, J.D. Elemental signatures in otoliths of hatchery rainbow trout (Oncorhynchus mykiss): Distinctiveness and utility for detecting origins and movement. Can. J. Fish. Aquat. Sci. 2009, 66, 513–524. [Google Scholar] [CrossRef]
- Wolff, B.A.; Johnson, B.M.; Landress, C.M. Classification of hatchery and wild fish using natural geochemical signatures in otoliths, fin rays, and scales of an endangered catostomid. Can. J. Fish. Aquat. Sci. 2013, 70, 1775–1784. [Google Scholar] [CrossRef]
- Ciepiela, L.R.; Walters, A.W. Life-history variation of two inland salmonids revealed through otolith microchemistry analysis. Can. J. Fish. Aquat. Sci. 2019, 76, 1971–1981. [Google Scholar] [CrossRef]
- Wolff, B.A.; Johnson, B.M.; Breton, A.R.; Martinez, P.J.; Winkelman, D.L. Origins of invasive piscivores determined from the strontium isotope ratio (87Sr/86Sr) of otoliths. Can. J. Fish. Aquat. Sci. 2012, 69, 724–739. [Google Scholar] [CrossRef] [Green Version]
- Thorrold, S.R.; Shuttleworth, S. In situ analysis of trace elements and isotope ratios in fish otoliths using laser ablation sector field inductively coupled plasma mass spectrometry. Can. J. Fish. Aquat. Sci. 2000, 57, 1232–1242. [Google Scholar] [CrossRef]
- Ranaldi, M.M.; Gagnon, M.M. Zinc incorporation in the otoliths of juvenile pink snapper (Pagrus auratus Forster): The influence of dietary versus waterborne sources. J. Exp. Mar. Biol. Ecol. 2008, 360, 56–62. [Google Scholar] [CrossRef]
- Gillanders, B.M. Temporal and spatial variability in elemental composition of otoliths: Implications for determining stock identity and connectivity of populations. Can. J. Fish. Aquat. Sci. 2002, 59, 669–679. [Google Scholar] [CrossRef]
- Ciepiela, L.R.; Walters, A.W. Quantifying 87Sr/86Sr temporal stability and spatial heterogeneity for use in tracking fish movement. Can. J. Fish. Aquat. Sci. 2019, 76, 928–936. [Google Scholar] [CrossRef]
- Elsdon, T.S.; Gillanders, B.M. Interactive effects of temperature and salinity on otolith microchemistry: Challenges for determining environmental histories of fish. Can. J. Fish. Aquat. Sci. 2002, 59, 1796–1808. [Google Scholar] [CrossRef]
- Sturrock, A.M.; Trueman, C.N.; Milton, J.A.; Waring, C.P.; Cooper, M.J.; Hunter, E. Physiological influences can outweigh environmental signals in otolith microchemistry research. Mar. Ecol.-Prog. Ser. 2014, 500, 245–264. [Google Scholar] [CrossRef] [Green Version]
- Tanner, S.E.; Reis-Santos, P.; Cabral, H.N. Otolith chemistry in stock delineation: A brief overview, current challenges and future prospects. Fish. Res. 2016, 173, 206–213. [Google Scholar] [CrossRef]
- Nazir, A.; Khan, M.A. Using otoliths for fish stock discrimination: Status and challenges. Acta Ichthyol. Piscat. 2021, 51, 199–218. [Google Scholar] [CrossRef]
- Aguilar, A.; Banks, J.D.; Levine, K.F.; Wayne, R.K. Population genetics of northern pike (Esox lucius) introduced into Lake Davis, California. Can. J. Fish. Aquat. Sci. 2005, 62, 1589–1599. [Google Scholar] [CrossRef]
- Muhlfeld, C.C.; Bennett, D.H.; Steinhorst, R.K.; Marotz, B.; Boyer, M. Using bioenergetics modeling to estimate consumption of native juvenile salmonids by nonnative northern pike in the Upper Flathead River system, Montana. N. Am. J. Fish. Manag. 2008, 28, 636–648. [Google Scholar] [CrossRef]
- Dunker, K.; Massengill, R.; Bradley, P.; Jacobson, C.; Swenson, N.; Wizik, A.; DeCino, R. A decade in review: Alaska’s adaptive management of an invasive apex predator. Fishes 2020, 5, 12. [Google Scholar] [CrossRef]
- Nesler, T.P. Interactions between Endangered Fishes and Introduced Gamefishes in the Yampa River, Colorado, 1987–1991; Colorado Division of Wildlife: Fort Collins, CO, USA, 1995. [Google Scholar]
- Zelasko, K.A.; Bestgen, K.R.; Hawkins, J.A.; White, G.C. Evaluation of long-term predator removal program: Abundance and population dynamics of invasive northern pike in the Yampa River, Colorado. Trans. Am. Fish. Soc. 2016, 145, 1153–1170. [Google Scholar] [CrossRef]
- Johnson, B.M.; Martinez, P.J.; Hawkins, J.A.; Bestgen, K.R. Ranking predatory threat by nonnative fishes in the Yampa River, Colorado, via bioenergetics modeling. N. Am. J. Fish. Manag. 2008, 28, 1941–1953. [Google Scholar] [CrossRef]
- Rogers, K.B.; Atkinson, B.F.; Orabutt, D.E. Catamount Reservoir: Fish Community Summary; Colorado Division of Wildlife: Steamboat Springs, CO, USA, 2005. [Google Scholar]
- Bauch, N.J.; Moore, J.L.; Schaffrath, K.R.; Dupree, J.A. Water-Quality Assessment and Macroinvertebrate Data for the Upper Yampa River Watershed, Colorado, 1975 through 2009; U.S. Geological Survey Scientific Investigations Report 2012–5214; U.S. Geological Survey: Reston, VA, USA, 2012.
- Walther, B.D.; Thorrold, S.R. Water, not food, contributes the majority of strontium and barium deposited in the otoliths of a marine fish. Mar. Ecol.-Prog. Ser. 2006, 311, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Feyrer, F.; Hobbs, J.; Baerwald, M.; Sommer, T.; Yin, Q.; Clark, K.; May, B.; Bennett, W. Otolith microchemistry provides information complementary to microsatellite DNA for a migratory fish. Trans. Am. Fish. Soc. 2007, 136, 469–476. [Google Scholar] [CrossRef]
- Stoeser, D.B.; Green, G.N.; Morath, L.C.; Heran, W.D.; Wilson, A.B.; Moore, D.W.; Van Gosen, B.S. Preliminary integrated geologic map databases for the United States, Central States—Montana, Wyoming, Colorado, New Mexico, North Dakota, South Dakota, Nebraska, Kansas, Oklahoma, Texas, Iowa, Missouri, Arkansas, and Louisiana: U.S. Geological Survey Open-File Report 2005–1351, version 1.2. 2005; updated December 2007. Available online: http://pubs.usgs.gov/of/2005/1351/ (accessed on 1 June 2012).
- Tyus, H.M.; Beard, J.M. Esox lucius (Esocidae) and Stizostedium vitreum (Percidae) in the Green River basin, Colorado and Utah. Great Basin Nat. 1990, 50, 33–39. [Google Scholar]
- Martinez, A.; Felt, B.; Dewhirst, J.; Eyre, T.; Martin, L. FY 2020 Annual Report Project Number C-20; Upper Colorado River Endangered Fish Recovery Program: Meeker, CO, USA, 2020. [Google Scholar]
- Orabutt, D.E. Northern Pike in Selected Colorado Trout Reservoirs. Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2006. [Google Scholar]
- Hill, C.G. Dynamics of Northern Pike Spawning and Nursery Habitat in the Yampa River, Colorado. Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2004. [Google Scholar]
- Martin, L.M. Middle Yampa River Northern Pike Removal and Evaluation #98A-1, Colorado River Recovery Program FY 2005 Annual Project Report; Colorado Division of Wildlife: Fort Collins, CO, USA, 2005. [Google Scholar]
- Craig, J.F. Pike: Biology and Exploitation; Chapman & Hall: London, UK, 1996. [Google Scholar]
- Rogers, K.B.; (Colorado Parks and Wildlife, Steamboat Springs, CO, USA). Personal communication, 2001.
- Whitledge, G.W.; Johnson, B.M.; Martinez, P.J.; Martinez, A.M. Sources of nonnative centrarchids in the Upper Colorado River revealed by stable isotope and microchemical analyses of otoliths. Trans. Am. Fish. Soc. 2007, 1036, 1263–1275. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.A.; Ridley, W.I.; Koenig, A.E. Development of sulfide calibration standards for the laser ablation inductively-coupled plasma mass spectrometry technique. J. Anal. At. Spectrom. 2002, 17, 406–409. [Google Scholar] [CrossRef]
- Longerich, H.P.; Jackson, S.E.; Gunther, D. Laser ablation-inductively coupled plasma-mass spectrometric transient signal data acquisition and analyte concentration calculation. J. Anal. At. Spectrom. 1996, 11, 899–904. [Google Scholar] [CrossRef]
- Pracheil, B.M.; George, R.; Chakoumakos, B.C. Significance of otolith calcium carbonate crystal structure diversity to microchemistry studies. Rev. Fish. Biol. Fish. 2019, 29, 569–588. [Google Scholar] [CrossRef]
- Yoshinaga, J.; Nakama, A.; Morita, M.; Edmonds, J.S. Fish otolith reference material for quality assurance of chemical analyses. Mar. Chem. 2000, 69, 91–97. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 5th ed.; Allyn and Bacon: Boston, MA, USA, 2007. [Google Scholar]
- Quinn, G.P.; Keogh, M.J. Experimental Design and Data Analysis for Biologists; University Press: New York, NY, USA, 2002. [Google Scholar]
- Turkheimer, F.E.; Hinz, R.; Cunningham, V.J. On the undecidability among kinetic models: From model selection to model averaging. J. Cereb. Blood Flow Metab. 2003, 23, 490–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, B.K.; Reiman, B.E.; Clayton, J.L.; Horan, D.L.; Jones, C.M. Relationship between water, otolith and scale chemistries of westslope cutthroat trout from Coeur d’Alene River, Idaho: The potential application of hard-part chemistry to describe movements in freshwater. Trans. Am. Fish. Soc. 2003, 132, 409–424. [Google Scholar] [CrossRef]
- Brazner, J.C.; Campana, S.E.; Tanner, D.K.; Schram, S.T. Habitat fingerprints from Lake Superior coastal wetlands derived from elemental analysis of yellow perch otoliths. Trans. Am. Fish. Soc. 2004, 133, 692–704. [Google Scholar] [CrossRef]
- Bronte, C.R.; Hesselberg, R.J.; Shoesmith, J.A.; Hoff, M.H. Discrimination among spawning concentration of Lake Superior lake herring based on trace element profiles in sagittae. Trans. Am. Fish. Soc. 1996, 125, 852–859. [Google Scholar] [CrossRef]
- Carlson, A.K.; Fincel, M.J.; Graeb, B.D.S. Otolith chemistry indicates walleye movement and entrainment in a large serial reservoir system. Fish. Manag. Ecol. 2017, 24, 217–229. [Google Scholar] [CrossRef]





| Canonical Correlations | ||||
|---|---|---|---|---|
| Year | Eigenvalue | 86Sr | 137Ba | 55Mn |
| 2005 | 2.24 | 0.55 | −0.46 | −0.28 |
| 2006 | 0.50 | 0.37 | 0.76 | 0.66 |
| 2007 | 1.11 | 0.81 | 0.54 | 0.34 |
| Model | AICc | ΔAICc | wi |
|---|---|---|---|
| Intercept, site, year, and site x year | −2041.8 | 0 | 0.823 |
| Intercept, site, year | −2038.2 | 3.6 | 0.136 |
| Intercept and site | −2035.8 | 6.0 | 0.041 |
| Intercept and year | −1315.0 | 726.8 | 0.000 |
| Intercept | −1299.2 | 742.6 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitzpatrick, R.M.; Winkelman, D.L.; Johnson, B.M. Using Isotopic Data to Evaluate Esox lucius (Linnaeus, 1758) Natal Origins in a Hydrologically Complex River Basin. Fishes 2021, 6, 67. https://0-doi-org.brum.beds.ac.uk/10.3390/fishes6040067
Fitzpatrick RM, Winkelman DL, Johnson BM. Using Isotopic Data to Evaluate Esox lucius (Linnaeus, 1758) Natal Origins in a Hydrologically Complex River Basin. Fishes. 2021; 6(4):67. https://0-doi-org.brum.beds.ac.uk/10.3390/fishes6040067
Chicago/Turabian StyleFitzpatrick, Ryan M., Dana L. Winkelman, and Brett M. Johnson. 2021. "Using Isotopic Data to Evaluate Esox lucius (Linnaeus, 1758) Natal Origins in a Hydrologically Complex River Basin" Fishes 6, no. 4: 67. https://0-doi-org.brum.beds.ac.uk/10.3390/fishes6040067