Analysis of the Parameters Required to Properly Define Nanofluids for Heat Transfer Applications
Abstract
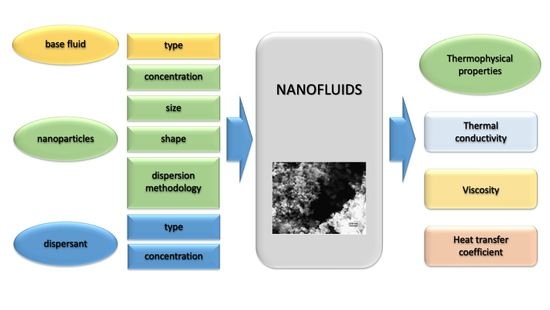
:1. Introduction
2. Nanofluid Parameters Influencing Thermophysical Properties
2.1. Methods of Preparation
2.1.1. Two-Step Method
2.1.2. One-Step Method
2.2. Effect of Nanoparticle Concentration and Morphology on Thermophysical Properties
2.2.1. Nanoparticle Concentration
2.2.2. Nanoparticle Size
2.2.3. Nanoparticle Shape
2.3. Effect of Dispersion Methodology on Thermophysical Properties
2.3.1. Effect of Dispersants
2.3.2. Effect of Cluster Reduction by Ultrasonication, Milling, High Pressure Homogenization
2.3.3. Effect of pH and Zeta Potential
3. A Case Study: Thermophysical Properties of TiO2-Water Nanofluids as a Function of Preparation Parameters
3.1. Analysis of Parameters Characterizing the TiO2-Water Nanofluids
3.1.1. Nanoparticles Concentration
3.1.2. Nanoparticles Shape
3.1.3. Nanoparticle Size
3.1.4. Dispersant Type and Concentration
3.1.5. Dispersion Methodology
3.1.6. pH and Zeta Potential
3.1.7. Discussion
| TiO2 Nanoparticles | Dispersant | Dispersion Methodology | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. | Concentration | Shape | Nominal Size (nm) | Cluster Size (nm) | Particle/Cluster monitoring | Type | Concentration | Technique | Time | Power/Frequency | pH | Zeta Potential |
| [44] | 0.24%, 0.6%, 1.18% vf (1–2.5–4.9% wf) | ND | 20 | 500 after sonication; 120 after dyno-mill; 95 further dyno-mill) (at 0.6% vf 95 nm, 145 nm, 210 nm | X-ray; SEM; DLS nanosizer | ND | ND | Ultrasonication for breaking down agglomerates before measurement; dyno mill for breaking; further dyno-mill | 30 m (+ 30 m + several hours) | ND | 11 | −40 mV |
| [38] | 1–5% vf | spherical | 15 | ND | ND | CTAB | 0.1 mM | ultrasonic dismembrator | ND | ND | ND | ND |
| [48] | 0.05–5% vf | spherical | 33 (anatase) | ND | XRD; EDX; FESEM | No surfactant; CTAB; SDS; Span 80 | ND | magnetic stirrer + ultrasonic | 2 h | ND | ND | ND |
| [51] | 3–9%; 9%; 9% wt | spherical | SEM 30; 30; 30 | DLS 140; 200; 90 | SEM; DLS | No surfactant; polycarboxylate; trioxadecane acid; | high-energy tip sonication | 15 min | ND | 7.2; 7.5; unknown | ND | |
| [27] | 1–5% | ND | 34 | 170 nm | SEM | NO surfactant | 0 | high shear homogenizer | 50 min (optimized) | ND | 3 | Z as a function of pH |
| [28] | 0.27–1.39% | ND | 32 | 100–200 (analytical discussion) | Laser diffraction technique; SEM; XRD | ND | ND | stirred bead milling/high shear homogenizer/ultrasonication | 12 h/15 min/ND | 1440 rpm/10,000–18,000rpm/130 W–20 Hz | ND | 42 mV |
| [54] | 0.5–5% vf | rod | 10 × 40 | TEM, particle size analyzer | Oleic acid | 0.01–0.02% | ultrasonication | 8–10 h | ND | 6.8–6.2 | ND | |
| spherical | 15 | cluster analysis | CTAB | |||||||||
| [55] | 10–20–40% mf | spherical | 40 | ND | TEM | No surfactant | 0 | ultrasonication | ND | ND | ND | ND |
| [56] | 0.2–3% vf | ND | 21 | ND | ND | No surfactant | 0 | ultrasonication | ND | ND | ND | ND |
| [57] | 0.5–4% vf | ND | 26 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| [58] | 0.1–1% vf | ND | 25 | ND | TEM; XRD | No surfactant | 0 | ultrasonication | ND | 700 W/20 kHz | ND | ND |
| [59] | 0.01–1% mf | spherical | 21 | around 100 nm | DLS | PEG600 | 2 disp:1 np | high pressure homogenization | − | ND | ND | 37–43 |
| [62] | 0.5–2.5% vf | spherical | 40 | ND | TEM | No surfactant | 0 | sonication | ND | ND | ND | ND |
| [61] | 0.2–1.2% | spherical | 25 | ND | SEM, DLS | No surfactant | 0 | Ultrasonication/shear homogenizer/medium-mill | ND | ND | high | ND |
| [62] | 1–10–20–35% mf | spherical | ND | 72–76 | DLS | Acetic acid | 1–5% | commercial (dilution with ultrasonication) | ND | ND | 1.86–3.07 | 55 mV |
| [63] | 0.1–2% | ND | 10–40 | 147–207 | TEM, HRTEM; DLS | CTAB; acetic acid | 1:10 nps | Stirring + ultrasonic | 2 h + 2−3 h | ND | 2.8–3.7 (AA); 3.9–4.9 (CTAB) | ND |
| [64] | 0.99–4% | ND | 27 | ND | visual | No surfactant | 0 | high speed mixer | 2 h | ND | 10 (analytical discussion) | ND |
| [65] | 0.2–2% (40% diluted) | spherical | 21 | ND | TEM | ND | ND | stirring for dilution; ultrasonication for cluster breaking | sonication 2 h | ND | 6.5–7.5 | ND |
| [66] | 0.1–4% | ND | 10 | ND | ND | CMC | 0.5% mf | ultrasonication | 1 h | ND | ND | |
| [67] | 0.89–6% | spherical | 5/5–15/30–50 | ND | NO | No surfactant; PVP | 1% mf | ultrasonication | 2 h; ND | ND | ND | ND |
3.2. Analysis Of Experimental Thermal Conductivity Enhancement
Effect of Measurement Technique
4. Conclusions
- (a)
- to highlight, through a selective analysis of the literature, the importance of the various parameters that characterize the complexity of nanofluids and their influence on thermophysical properties;
- (b)
- to evaluate, through the systematic comparison of the information reported in the literature for a case study, whether these parameters are always reported, in order to allow an adequate definition of the nanofluid and the interpretation of the results obtained from the property measurements.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Benos, L.; Spyrou, L.A.; Sarris, I.E. Development of a new theoretical model for blood-CNTs effective thermal conductivity pertaining to hyperthermia therapy of glioblastoma multiform. Comput. Methods Prog. Biomed. 2019, 172, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Karvelas, E.; Liosis, C.; Benos, L.; Karakasidis, T.; Sarris, I. Micromixing Efficiency of Particles in Heavy Metal Removal Processes under Various Inlet Conditions. Water 2019, 11, 1135. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.B.; Heris, S.Z.; Estellé, P. Experimental comparison between ZnO and MoS2 nanoparticles as additives on performance of diesel oil-based nano lubricant. Sci. Rep. 2020, 10, 5813. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.U.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles. In Proceedings of the International Mechanical Engineering Congress and Exhibition, San Francisco, CA, USA, 12–17 November 1995; pp. 99–106. [Google Scholar]
- Sidik, N.A.; Yazid, M.N.; Mamat, R. A review on the application of nanofluids in vehicle engine cooling system. Int. Commun. Heat Mass 2015, 68, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Villarejo, R.; Estellé, P.; Navas, J. Boron nitride nanotubes-based nanofluids with enhanced thermal properties for use as heat transfer fluids in solar thermal applications. Sol. Energy Mater. Sol. Cells 2020, 205, 110266. [Google Scholar] [CrossRef]
- Kulkarni, D.P.; Das, D.K.; Vajjha, R.S. Application of nanofluids in heating buildings and reducing pollution. Appl. Energy 2009, 86, 2566–2573. [Google Scholar] [CrossRef]
- Bhattad, A.; Sarkar, J.; Ghosh, P. Improving the performance of refrigeration systems by using nanofluids: A comprehensive review. Renew. Sustain. Energy Rev. 2018, 82, 3656–3669. [Google Scholar] [CrossRef]
- Bahiraei, M.; Heshmatian, S. Electronics cooling with nanofluids: A critical review. Energy Convers. Manag. 2018, 172, 438–456. [Google Scholar] [CrossRef]
- Gkountas, A.A.; Benos, L.T.; Nikas, K.-S.; Sarris, I.E. Heat transfer improvement by an Al2O3-water nanofluid coolant in printed-circuit heat exchangers of supercritical CO2 Brayton cycle. Therm. Sci. Eng. Prog. 2020, 20, 100694. [Google Scholar] [CrossRef]
- Saidur, R.; Leong, K.Y.; Mohammed, H.A. A review on applications and challenges of nanofluids. Renew. Sustain. Energy Rev. 2011, 15, 1646–1668. [Google Scholar] [CrossRef]
- Chakraborty, S.; Panigrahi, P.K. Stability of nanofluid: A review. Appl. Therm. Eng. 2020, 164, 115259. [Google Scholar] [CrossRef]
- Koca, H.D.; Doganay, S.; Turgut, A.; Tavman, I.H.; Saidur, R.; Mahbubul, I.M. Effect of particle size on the viscosity of nanofluids: A review. Renew. Sustain. Energy Rev. 2018, 82, 1664–1674. [Google Scholar] [CrossRef] [Green Version]
- Maheshwary, P.B.; Handa, C.C.; Nemade, K.R. A comprehensive study of effect of concentration, particle size and particle shape on thermal conductivity of titania/water based nanofluid. Appl. Therm. Eng. 2017, 119, 79–88. [Google Scholar] [CrossRef]
- Zhou, M.; Xia, G.; Li, J.; Chai, L.; Zhou, L. Analysis of factors influencing thermal conductivity and viscosity in different kinds of surfactant solutions. Exp. Therm. Fluid Sci. 2012, 36, 22–29. [Google Scholar]
- Timofeeva, E.V.; Routbort, J.L.; Singh, D. Particle shape effects on thermophysical properties of alumina nanofluids. J. Appl. Phys. 2009, 106, 014304. [Google Scholar] [CrossRef]
- Wang, X.-J.; Li, X.-F. Influence of pH on Nanofluids’ Viscosity and Thermal Conductivity. Chin. Phys. Lett. 2009, 26, 056601. [Google Scholar]
- Lee, D.; Kim, J.-W.; Kim, B.G. A new parameter to control heat transport in nanofluids: Surface charge state of the particle in suspension. J. Phys. Chem. B 2006, 110, 4323–4328. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Tung, S.; Schneider, E.; Xi, S. A review on development of nanofluid preparation and characterization. Powder Technol. 2009, 196, 89–101. [Google Scholar] [CrossRef]
- Gupta, M.; Singh, V.; Kumar, R.; Said, Z. A review on thermophysical properties of nanofluids and heat transfer applications. Renew. Sustain. Energy Rev. 2017, 74, 638–670. [Google Scholar] [CrossRef]
- Wen, D.; Lin, G.; Vafaei, S.; Zhang, K. Review of nanofluids for heat transfer applications. Particuology 2009, 7, 141–150. [Google Scholar] [CrossRef]
- Asadi, A.; Aberoumand, S.; Moradikazerouni, A.; Pourfattah, F.; Zyla, G.; Estellè, P.; Mahian, O.; Wongwises, S.; Nguyen, H.M.; Arabkooshar, A. Recent advances in preparation methods and thermophysical properties of oil-based nanofluids: A state-of-the-art review. Powder Technol. 2019, 352, 209–226. [Google Scholar] [CrossRef]
- Hafiz, M.A.; Babar, H.; Shah, T.R.; Sajid, M.U.; Qasim, M.A.; Javed, S. Preparation Techniques of TiO2 Nanofluids and Challenges: A Review. Appl. Sci. 2018, 8, 587. [Google Scholar] [CrossRef] [Green Version]
- Saini, A.; Kaur, H.; Sharma, S.; Gangacharyulu, D. Nanofluids: A Review Preparation, Stability, Properties and Applications. Int. J. Eng. Res. Technol. 2016, 5, 11–15. [Google Scholar]
- Kwak, K.; Kim, C. Viscosity and thermal conductivity of copper oxide nanofluid dispersed in ethylene glycol. Korea-Aust. Rheol. J. 2005, 17, 35–40. [Google Scholar]
- Wen, D.; Ding, Y. Natural Convective Heat Transfer of Suspensions of Titanium Dioxide Nanoparticles (Nanofluids). IEEE Trans. Nanotechnol. 2006, 5, 220–227. [Google Scholar]
- Silambarasan, M.; Manikandan, S.; Rajan, K.S. Viscosity and thermal conductivity of dispersions of sub-micron TiO2 particles in water prepared by stirred bead milling and ultrasonication. Int. J. Heat Mass Transf. 2012, 55, 7991–8802. [Google Scholar] [CrossRef]
- Li, X.F.; Zhu, D.S.; Wang, X.J.; Wang, N.; Gao, J.W.; Li, H. Thermal conductivity enhancement dependent pH and chemical surfactant for Cu-H2O nanofluids. Thermochim. Acta 2008, 469, 98–103. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Eastman, J.A. Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl. Phys. Lett. 2001, 78, 718–720. [Google Scholar]
- Lee, G.J.; Kim, C.K.; Lee, M.K.; Rhee, C.K. Characterization of ethylene glycol based TiO2 nanofluid prepared by pulsed wire evaporation (PWE) method. Rev. Adv. Mater. Sci. 2011, 28, 126–129. [Google Scholar]
- Lo, C.H.; Tsung, T.T.; Chen, L.C. Shape-controlled synthesis of Cu-based nanofluid using submerged arc nanoparticle synthesis system (SANSS). J. Cryst. Growth 2005, 277, 636–642. [Google Scholar] [CrossRef]
- Wu, Y.; Kao, M. Using TiO2 nanofluid additive for engine lubrication oil. Ind. Lubr. Tribol. 2011, 63, 440–445. [Google Scholar] [CrossRef]
- Huang, X.X.; Zhang, W.G. Study on successively preparation of nano-TiO2 ethanol colloids by pulsed laser ablation and fluorescence property. Appl. Surf. Sci. 2008, 254, 3403–3407. [Google Scholar] [CrossRef]
- Zhu, H.T.; Lin, Y.S.; Yin, Y.S. A novel one-step chemical method for preparation of copper nanofluids. J. Colloid Interface Sci. 2004, 277, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Prk, S.D.; Kang, S.; Bang, I.C.; Kim, J.H. Investigation of viscosity and thermal conductivity of SiC nanofluids for heat transfer applications. Int. J. Heat Mass. Transf. 2011, 54, 433–438. [Google Scholar] [CrossRef]
- Teng, T.P.; Hung, Y.H.; Teng, T.C.; Mo, H.E.; Hsu, H.G. The effect of alumina/water nanofluid particle size on thermal conductivity. Appl. Therm. Eng. 2010, 30, 2213–2218. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Investigations of thermal conductivity and viscosity of nanofluids. Int. J. Therm. Sci. 2008, 47, 560–568. [Google Scholar] [CrossRef]
- Sundar, L.S.; Singh, M.K.; Sousa, A.C.M. Investigation of thermal conductivity and viscosity of Fe3O4 nanofluid for heat transfer applications. Int. Commun. Heat Mass 2013, 44, 7–14. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Smith, D.S.; Yo, W.; France, D.M.; Singh, D.; Routbort, J.L. Particle size and interfacial effects on thermo-physical and heat transfer characteristics of water-based α-SiC nanofluids. Nanotechnology 2010, 21, 215703. [Google Scholar] [CrossRef]
- Paul, G.; Pal, T.; Manna, I. Thermo-physical property measurement of nano-gold dispersed water based nanofluids prepared by chemical precipitation technique. J. Colloid Interface Sci. 2010, 349, 434–437. [Google Scholar] [CrossRef]
- Kwek, D.; Crivol, A.; Duan, F. Effects of Temperature and Particle Size on the Thermal Property Measurements of Al2O3-Water Nanofluids. J. Chem. Eng. Data 2010, 55, 5690–5695. [Google Scholar] [CrossRef]
- Keblinski, P.; Phillpot, S.R.; Choi, S.U.S.; Eastman, J.A. Mechanisms of heat flow in suspensions of nano-sized particles (nanofluids). Int. J. Heat Mass 2002, 45, 855–863. [Google Scholar] [CrossRef]
- He, Y.; Jin, Y.; Chen, H.; Ding, Y.; Cang, D.; Lu, H. Heat transfer and flow behaviour of aqueous suspensions of TiO2 nanoparticles (nanofluids) flowing upward through a vertical pipe. Int. J. Heat Mass 2007, 50, 2272–2281. [Google Scholar] [CrossRef]
- Shalkevich, N.; Escher, W.; Burgi, T.; Michel, B.; Si-Ahmed, L.; Poulikakos, D. On the thermal conductivity of gold nanoparticle colloids. Langmuir 2010, 26, 663–670. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Desgranges, F.; Roy, G.; Galanis, N.; Marè, T.; Boucher, S.; Mintsa, H.A. Temperature and particle-size dependent viscosity data for water-based nanofluids—Hysteresis phenomenon. Int. J. Heat Fluid Flow 2007, 28, 1492–1506. [Google Scholar] [CrossRef]
- Mirmohammadi, S.A.; Behi, M.; Gan, Y.; Shen, L. Particle-shape-, temperature-, and concentration-dependent thermal conductivity and viscosity of nanofluids. Phys. Rev. E 2019, 99, 043109. [Google Scholar] [CrossRef] [Green Version]
- Saleh, R.; Putra, N.; Wibowo, R.E.; Septiadi, W.N.; Prakoso, S.P. Titanium dioxide nanofluids for heat transfer applications. Exp. Therm. Fluid Sci. 2014, 52, 19–29. [Google Scholar] [CrossRef]
- Hu, H.; Peng, H.; Ding, G. Nucleate pool boiling heat transfer characteristics of refrigerant/nanolubricant mixture with surfactant. Int. J. Refrig. 2013, 36, 1045–1055. [Google Scholar] [CrossRef]
- Alphonse, P.; Bleta, R.; Soules, R. Effect of PEG on rheology and stability of nanocrystalline titania hydrosols. J. Colloid Interface Sci. 2009, 337, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Jarahnejad, M.; Haghighi, E.B.; Saleemi, M.; NIkkam, N.; Khodabandeh, R.; Palm, B.; Toprak, M.S.; Muhammed, M. Experimental investigation on viscosity of water-based Al2O3 and TiO2 nanofluids. Rheol. Acta 2015, 54, 411–422. [Google Scholar] [CrossRef]
- Fedele, L.; Colla, L.; Bobbo, S.; Agresti, F. Experimental stability analysis of different water based nanofluids. Nanoscale Res. Lett. 2011, 6, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.-C.; Li, F.-C.; Zhou, W.-W.; He, Y.-R.; Jiang, C.-B. Experimental investigation on the thermal conductivity and shear viscosity of viscoelastic-fluid-based nanofluids. Int. J. Heat Mass Transf. 2012, 55, 3160–3166. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Enhanced thermal conductivity of TiO2—water based nanofluids. Int. J. Therm. Sci. 2005, 44, 367–373. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, H.; Fujii, M. Experimental Study on the Effective Thermal Conductivity and Thermal Diffusivity of Nanofluids. Int. J. Thermophys. 2006, 27, 569–580. [Google Scholar] [CrossRef]
- Turgut, A.; Tavman, I.; Chirtoc, M.; Schuchmann, H.P.; Sauter, C.; Tavman, S. Thermal Conductivity and Viscosity Measurements of Water-Based TiO2 Nanofluids. Int. J. Thermophys. 2009, 30, 1213–1226. [Google Scholar] [CrossRef]
- Wang, Z.I.; Tang, D.W.; Liu, S.; Zheng, X.H.; Araki, N. Thermal-Conductivity and Thermal-Diffusivity Measurements of Nanofluids by 3ω Method and Mechanism Analysis of Heat Transport. Int. J. Thermophys. 2007, 28, 1255–1268. [Google Scholar] [CrossRef]
- Yoo, D.-H.; Hong, K.S.; Yang, H.-S. Study of thermal conductivity of nanofluids for the application of heat transfer fluids. Thermochim. Acta 2007, 455, 66–69. [Google Scholar] [CrossRef]
- Bobbo, S.; Fedele, L.; Benetti, A.; Colla, L.; Fabrizio, M.; Pagura, C.; Barison, S. Viscosity of water based SWCNH and TiO2 nanofluids. Exp. Therm. Fluid Sci. 2012, 36, 65–71. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, H.; Fujii, M. Effective thermal conductivity and thermal diffusivity of nanofluids containing spherical and cylindrical nanoparticles. Exp. Therm. Fluid Sci. 2007, 31, 593–599. [Google Scholar] [CrossRef]
- Chen, H.; Witharana, S.; Jin, Y.; Kim, C.; Ding, Y. Predicting thermal conductivity of liquid suspensions of nanoparticles (nanofluids) based on rheology. Particuology 2009, 7, 151–157. [Google Scholar] [CrossRef]
- Fedele, L.; Colla, L.; Bobbo, S. Viscosity and thermal conductivity measurements of water-based nanofluids containing titanium oxide nanoparticles. Int. J. Refrig. 2012, 35, 1359–1366. [Google Scholar] [CrossRef]
- Das, P.; Mallik, A.K.; Ganguly, R.; Santra, A.K. Synthesis and characterization of TiO2-water nanofluids with different surfactants. Int. Commun. Heat Mass 2016, 75, 341–348. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 2007, 11, 151–170. [Google Scholar] [CrossRef]
- Duangthongsuk, W.; Wongwises, S. Measurement of temperature-dependent thermal conductivity and viscosity of TiO2-water nanofluids. Exp. Therm. Fluid Sci. 2009, 33, 706–714. [Google Scholar] [CrossRef]
- Hojjat, M.; Etemad, S.G.; Bagheri, R.; Thibault, J. Thermal conductivity of non-Newtonian nanofluids: Experimental data and modeling using neural network. Int. J. Heat Mass Trans. 2011, 54, 1017–1023. [Google Scholar] [CrossRef]
- Tertsinidou, G.J.; Tsolakidou, C.M.; Pantzali, M.; Assael, M.J.; Colla, L.; Fedele, L.; Bobbo, S.; Wakeham, W.A. New Measurements of the Apparent Thermal Conductivity of Nanofluids and Investigation of Their Heat Transfer Capabilities. J. Chem. Eng. Data 2017, 62, 491–507. [Google Scholar] [CrossRef] [Green Version]
- Tertsinidou, G.; Assael, M.J.; Wakeham, W.A. The Apparent Thermal Conductivity of Liquids Containing Solid Particles of Nanometer Dimensions: A Critique. Int. J. Thermophys. 2015, 36, 1367–1395. [Google Scholar] [CrossRef]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobbo, S.; Buonomo, B.; Manca, O.; Vigna, S.; Fedele, L. Analysis of the Parameters Required to Properly Define Nanofluids for Heat Transfer Applications. Fluids 2021, 6, 65. https://0-doi-org.brum.beds.ac.uk/10.3390/fluids6020065
Bobbo S, Buonomo B, Manca O, Vigna S, Fedele L. Analysis of the Parameters Required to Properly Define Nanofluids for Heat Transfer Applications. Fluids. 2021; 6(2):65. https://0-doi-org.brum.beds.ac.uk/10.3390/fluids6020065
Chicago/Turabian StyleBobbo, Sergio, Bernardo Buonomo, Oronzio Manca, Silvio Vigna, and Laura Fedele. 2021. "Analysis of the Parameters Required to Properly Define Nanofluids for Heat Transfer Applications" Fluids 6, no. 2: 65. https://0-doi-org.brum.beds.ac.uk/10.3390/fluids6020065